Figure 5.
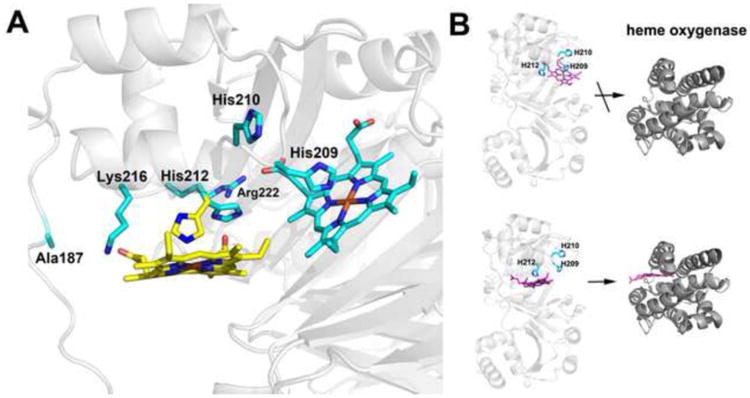
A) Modeling of two possible heme binding modes. Heme is modeled at both His 209 and His 212. His 209 is perfectly positioned to bind to heme while His 212 must adopt an alternative rotamer conformation to bind to heme. The rotamers observed in the crystal structure are cyan while the new rotamer conformation of His 212 required for heme ligation is yellow. We also checked to see if alternate rotamers of His 210 might be able to bind heme but His 210 extends out into solution and there is no rotamer position that orients His 210 into the pocket for heme coordination. B) Models emphasizing that heme transfer to heme oxygenase occurs only when His 212 is the ligand.
