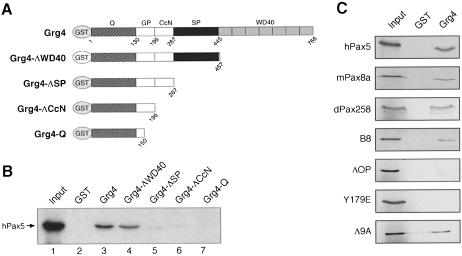
Fig. 4. In vitro binding of Pax5 to Grg4. (A) C-terminal deletions of GST–Grg4 proteins. (B) The SP domain of Grg4 interacts with Pax5. GST pull-down assays were used to study the interaction between in vitro translated, 35S-labeled Pax5 protein and GST (lane 2) or GST–Grg4 proteins (lanes 3–7) bound to glutathione–Sepharose. Lane 1 contained 10% of the Pax5 protein input. (C) The octapeptide of Pax5 mediates binding of Grg4. The 35S-labeled Pax proteins indicated were analyzed for binding to GST or GST–Grg4. The input lane contained ∼10% of the total Pax protein used in each assay.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.