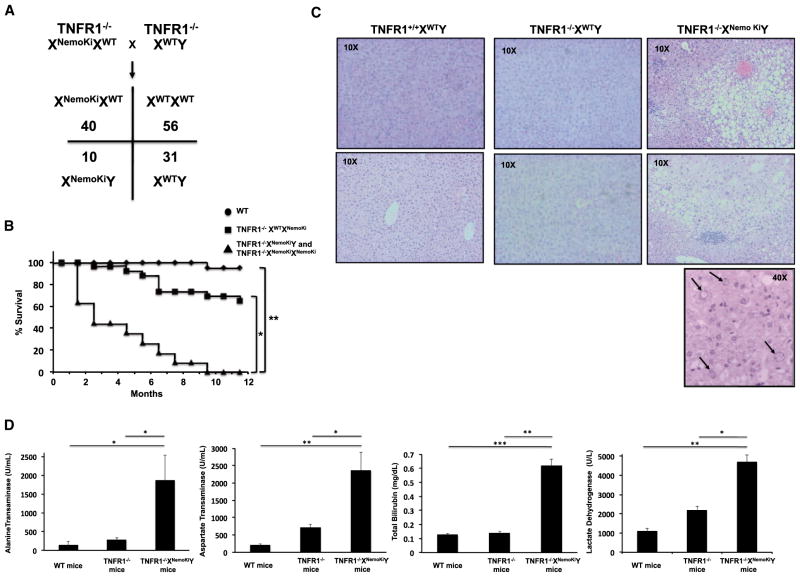
Figure 4. Mating onto a TNFR1-Deficient Background Rescues Embryonic Lethality in Nonubiquitinatable NEMO Mice, but Causes Steatohepatitis and Increased Mortality.
(A) C57BL/6 TNFR1−/− mice were mated with TNFR1+/+XWTXNemoKi mice and the resulting TNFR1+/−XWTXNemoki mice were mated with TNFR1−/−XWTY mice to obtain TNFR1−/−XWTXNemoKi mice, which were further mated with TNFR1−/−XWTY to generate TNFR1−/−XNemoKiY mice. The Mendelian ratios of this mating are shown. In a WT TNFR1 background, we did not obtain pure NEMO knockin mice; however, when TNFR1 was deleted, we obtained male nonubiquitinatable NEMO knockin mice, suggesting that TNFR1 deletion complements the nonubiquitinatable NEMO defect.
(B) Kaplan-Maier curves showing significantly decreased lifespans in the TNFR1−/−XNemoKiY and TNFR1−/−XNemoKiXNemoKi mice.
(C) Histologic evidence of steatohepatitis in the TNFR1−/−XNemoKiY mice, including macro- and microvesicular steatosis, inflammatory infiltrates most prominent in zone 3 of the liver, and prominent nucleolar vacuolization (lower panel, nucleolar vacuolization is shown by arrows). These are all features of NASH.
(D) Elevated liver function tests in the TNFR1−/−XNemoKiY mice, indicating significant hepatocyte injury. SEM, *p < 0.05, **p < 0.01, ***p < 0.001.