Figure 2.
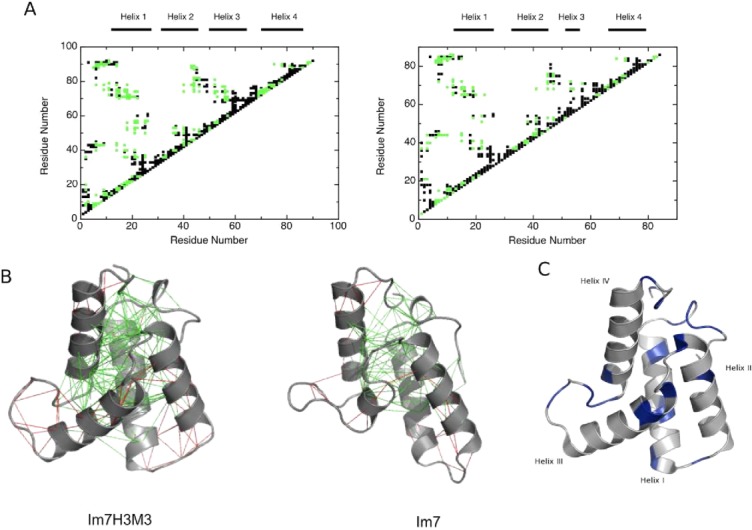
(A) Frustration contact maps of Im7H3M3 (left) and wild-type Im7 (right), with highly frustrated contacts coloured black and minimally frustrated coloured green. The frustration index (Fij)21 gives the energetic fitness for a given set of residues to interact. An interaction is minimally frustrated when Fij > 0.78 and highly frustrated when Fij < −1. (B) Configurational frustration network interactions represented on atomic structures of Im7H3M3 (PDB code: 2K0D) and wild-type Im7 (PDB code: 1AYI). A cluster of minimally frustrated contacts (green) defines the core of the protein, which involves all the α-helices, with highly frustrated contacts shown in red. (C) Backbone fold of Im7H3M3 with the residues exhibiting Rex terms derived from model-free analyses (see below) shown in blue. Residues without Rex terms are coloured grey. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
