Figure 6.
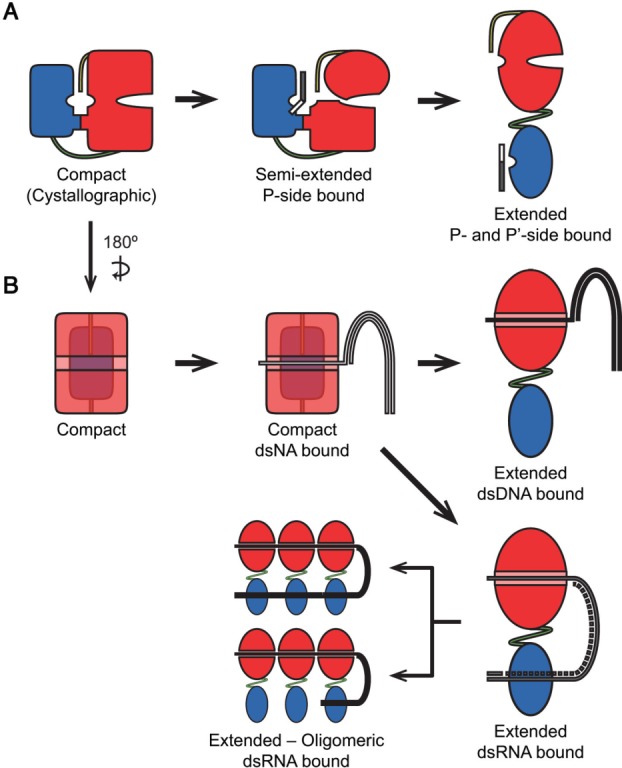
Possible models unifying our experimental results and previous observations on relations between HCV NS3/4A conformational states and protease and helicase function. (A) Binding of the P-side of a substrate induces conformation change from the closed conformation to a semiextended conformation. Conformational switch from the proposed semi-extended conformation to the extended conformation depends on the stability of the direct interface and the protease is fully active in the extended conformation. (B) Binding to the single-stranded region of a nucleic acid (either DNA or RNA) occurs in the closed conformation. For dsDNA, activation of the protein was modulated by interface mutations significantly, possibly due to the alteration of the dynamic interaction between the domains through the protease-helicase interface. For dsRNA, activity is independent of the interface, and protease-RNA association acts a factor modulating the conformational transition. The protease domain potentially associates with the dsRNA as a clamp. When the protein oligomerizes on dsRNA, two possible arrangements for RNA-protease interaction is possible – either all the protease domains or only the domain on the leading monomer associate with dsRNA.
