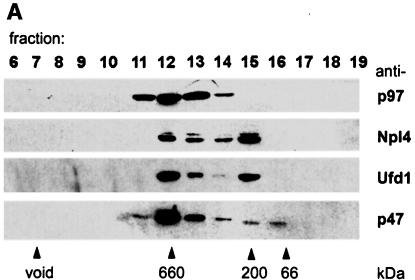
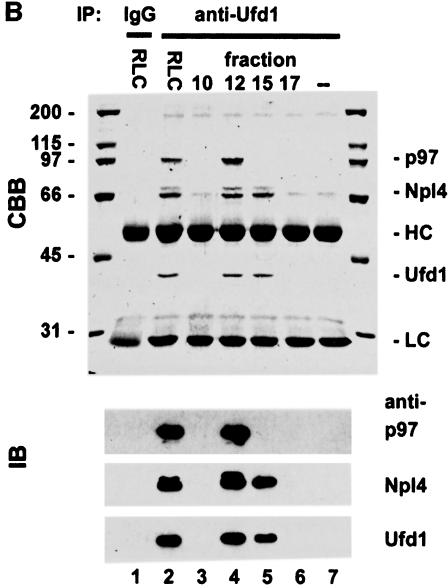
Fig. 5. Cytosol contains two independent complexes: a binary Ufd1/Npl4 and a ternary p97/Ufd1/Npl4 complex. (A) Rat liver cytosol (RLC) was fractionated by gel filtration on a Superose 6 column. Aliquots were separated by SDS–PAGE and p97, and its binding proteins analysed by immunoblotting. Almost all the p47 comigrated with the p97 peak at 600–800 kDa. In contrast, Npl4 and Ufd1 ran together exhibiting a biphasic distribution, the first peak co-migrating with p97, the second at ∼200 kDa. (B) Immunoprecipitation of Ufd1 complexes from RLC gel filtration fractions. Total RLC and selected gel filtration fractions identified in (A) were subjected to low stringency immunoprecipitation with a monoclonal anti-Ufd1 antibody. As controls, immunoprecipitation was performed from total RLC using non-immune mouse IgG (lane 1) and from buffer using anti-Ufd1 (lane 7). Precipitates were analysed by SDS–PAGE followed by Coomassie Blue-staining (CBB) or immunoblotting (IB) using specific rabbit antibodies. Npl4 and p97 were both co-immunoprecipitated with Ufd1 from total RLC (lane 2). When precipitated from the high molecular weight peak (lane 4), Ufd1 pulled down both Npl4 and p97, whereas precipitation from the 200 kDa peak (lane 5), bound only Npl4. Note that Ufd1 was not precipitated from fraction 17 (lane 6) where monomeric Ufd1 would be expected. HC, IgG heavy chain; LC, IgG light chain.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.