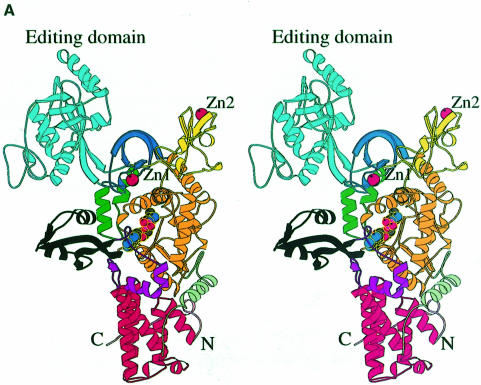
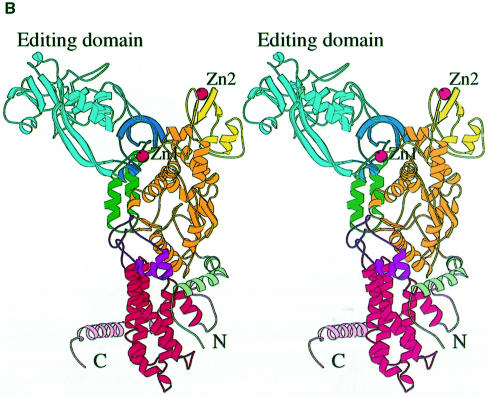
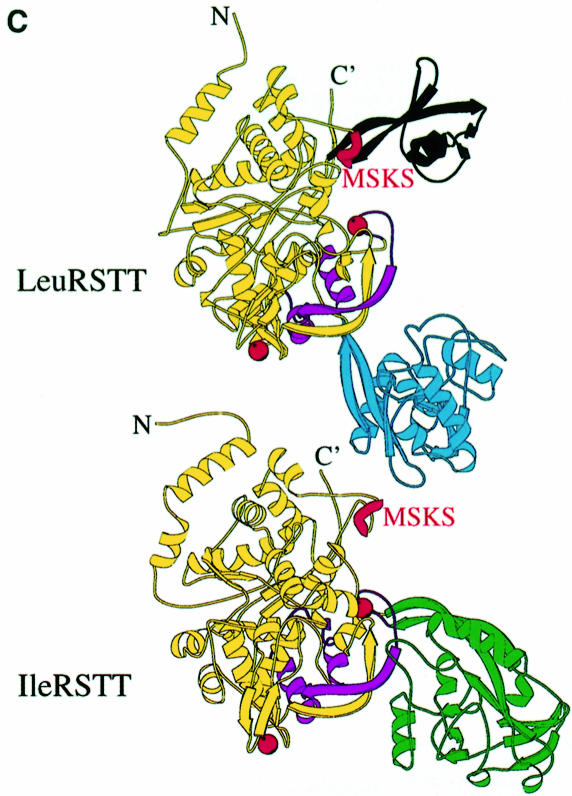
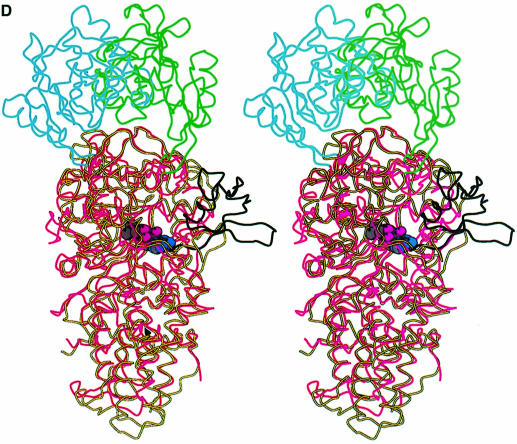
Fig. 1. (A) Stereo ribbon diagram of the structure of the complex between T.thermophilus leucyl-tRNA synthetase and a leucyl-adenylate analogue. The domains are coloured as follows: N-terminal extension (pale green), catalytic domain (Rossmann-fold, orange), ZN-1 domain (blue), helical hairpin insertion (green), editing domain (cyan), ZN-2 domain (yellow), leucyl-specific insertion domain (black), connecting module (purple), anti-codon binding domain (red) and C-terminal extension (pink). The zinc atoms are shown as red balls and the leucyl-adenylate analogue as a space-filling model in the centre. The N- and C-termini are marked. (B) Stereo ribbon diagram of the structure of the T.thermophilus isoleucyl-tRNA synthetase. Colouring as in (A). Note that in IleRS the ZN-1 domain is split by the inserted editing domain [see (C) and Figure 2]. (C) Comparison of point of insertion of extra domains in T.thermophilus leucyl- (top) and isoleucyl- (bottom) tRNA synthetases. For clarity, the enzymes are C-terminally truncated just before the anti-codon binding domain (C′). The purple polypeptide segment is homologous in each enzyme and contains the second half of the ZN-1 binding site [see also (A)]. In leucyl-tRNA synthetase, it precedes the editing domain (cyan), whereas in isoleucyl-tRNA synthetase it follows the editing domain (green). The disposition of the leucyl-specific insertion domain is shown in black. It is inserted just before the KMSKS loop (red segment in both enzymes). (D) Stereo view of the superposition of Cα traces of LeuRSTT and IleRSTT showing different points of insertion of the editing domain and the near 180° difference in rotational orientation. LeuRSTT is red with editing domain cyan, leucine-specific domain black and LeuAMS in the active site. IleRSTT is yellow with editing domain green. Considering the red and yellow traces, 437 Cα positions superpose within a cut-off of 3.8 Å with 115 identities and root-mean-square deviation (r.m.s.d.) of 1.99 Å. Superposing the two editing domains, the equivalent figures are 129 Cα positions with 37 identities and r.m.s.d. of 1.36 Å. In all, 69.5% of the 814 ordered residues in LeuRSTT can be superposed within 3.8 Å of equivalent residues in IleRSTT with 27% of these residues being identical.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.