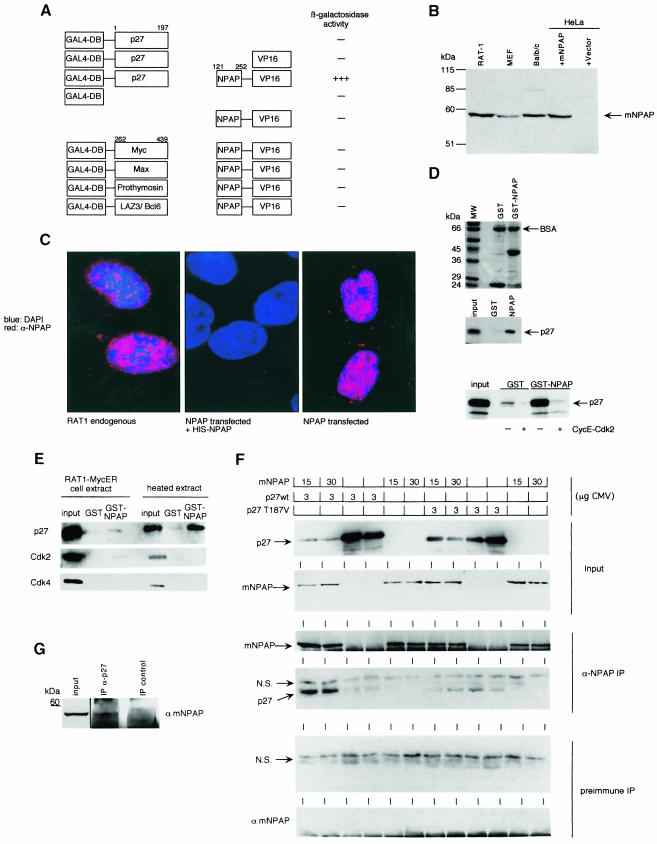
Fig. 1. Identification of mNPAP60 as a p27-interacting protein. (A) Summary of yeast two-hybrid interaction data. Plasmids expressing the indicated chimeras were transformed into yeast strains and β-galactosidase activity was determined by a semi-quantitative filter assay. (B) Western blot documenting the expression of endogenous mNPAP60 in rodent fibroblast cell lines (left lanes). The right lanes contain extracts from HeLa cells transfected with vector alone or with an expression vector encoding mNPAP60. The antibody raised against mouse NPAP does not cross-react with human NPAP. (C) Immunofluorescence of cultured RAT1 cells using an affinity-purified antiserum raised against mNPAP60 (left). The right panel shows the localization of mNPAP60 expressed by transient transfection in HeLa cells. The middle panel shows a control after pre-incubation of the antiserum with a GST–mNPAP60 fusion protein. The pictures are a false-colour superimposition of the 4′,6-diamidino-2-phenylindole-stained nuclei (coloured in blue) and anti-mNPAP60 fluorescence (coloured in red). (D) In vitro binding of 35S-labelled p27 to GST–mNPAP60(121–252). The upper panel shows Coomassie-stained gels of the input into the in vitro binding reactions together with a molecular weight marker. BSA was added to reduce background binding. The middle panel shows a fluorography demonstrating specific binding of p27 to GST–mNPAP60. The lower panel shows reduced binding of p27 to GST–mNPAP60 after pre-incubation of p27 with cyclin E–Cdk2 complexes purified from baculovirus. The input lanes correspond to 10% of the total amount of p27 loaded on to the GST beads. (E) In vitro binding reactions from cell lysates. Detergent lysates from RAT1-MycER cells were prepared with or without heat treatment and incubated with GST–mNPAP60 as above. Western blots of the input lanes (10% of loading) and the recovered beads are shown. (F) Co-immunoprecipitation of p27 with anti-mNPAP60 antibodies. HeLa cells were transfected with the expression plasmids indicated, lysates prepared and immunoprecipitated with either antiserum against mNPAP60 or preimmune serum. Shown are Western blots probed with the antibodies indicated. N.S. reflects a non-specific band present in all immunoprecipitates. mNPAP60 migrates just above the IgG heavy chain on SDS gels; since the same antibody is used for both immunoprecipitation and Western blotting, the heavy chain is visible below the mNPAP60 bands in the immunoprecipitations. (G) Co-immunoprecipitation of endogenous mNPAP60 with anti-p27, but not with control antibodies from lysates of NIH 3T3 cells. The input represents 5% of cell extract used for the precipitation.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.