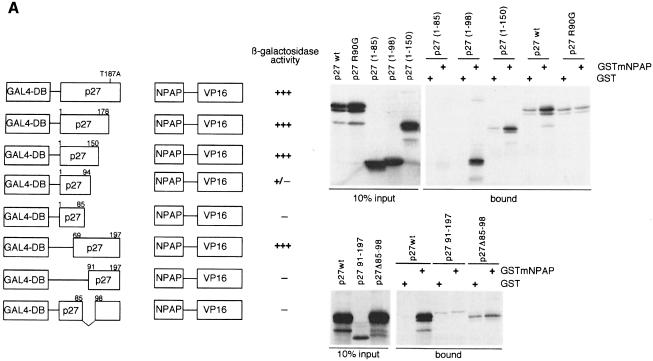
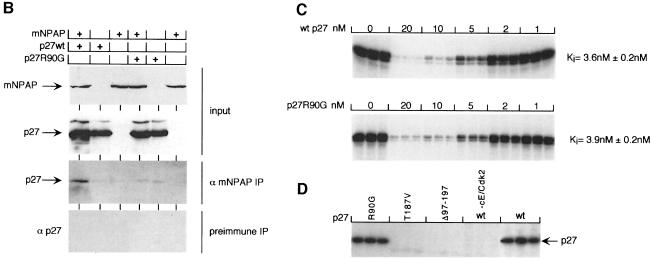
Fig. 2. Isolation of a mNPAP60 loss-of-interaction mutant of p27. (A) Summary of two-hybrid data (left) and in vitro interaction assays (right) documenting the interaction of C-terminal, N-terminal and internal deletion mutants of p27 with mNPAP60. The assays were carried out as described in the legend to Figure 1. p27Δ91–197 lacks three of five methionines and is, therefore, labelled more weakly in these experiments. The right panel also documents the reduced interaction of the p27R90G mutant with GST–mNPAP60 in vitro. (B) Western blot documenting co-immunoprecipitation of p27wt, but not of p27R90G after co-expression with mNPAP60 in HeLa cells. Assays were carried out as described in the legend to Figure 1. (C) Inhibition of cyclin E–Cdk2 kinase activity by increasing amounts of p27. His-tagged recombinant p27wt and p27R90G proteins were purified and incubated with cyclin E–Cdk2 complexes isolated from baculovirus lysates. Histone H1 was used as a substrate. Triplicate assays are shown and were used to calculate the Ki values shown on the right. (D) The proteins indicated were purified and equal amounts subjected to phosphorylation by immunoprecipitated cyclin E–Cdk2 kinase. Shown is an autoradiogram of triplicate assays. The control designated ‘-cE/Cdk2’ contains p27wt protein and a mock precipitate using no antibody.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.