Fig. 1. Location of the general factors in the Pol II PIC.
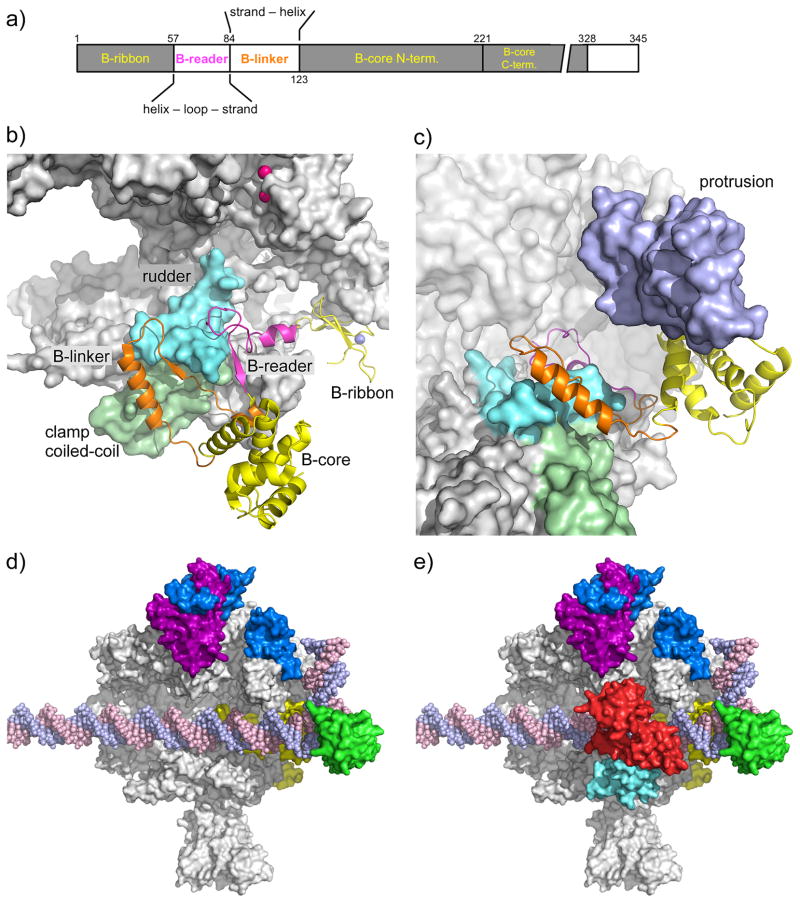
a) TFIIB domain organization. The domain color code (colored lettering) is used in panels a–c.
b) Pol II-TFIIB complex [19]. TFIIB is shown as a cartoon, Pol II as a grey surface. Rpb2 is removed for clarity. The Rpb1 rudder (cyan) and clamp coiled-coil (light green) are highlighted. The TFIIB Zn-atom is shown as blue sphere, the two Mg-atoms in the Pol II active site are shown as pink spheres.
c) TFIIB binding to Pol II results in ordering of the Rpb2 protrusion. To visualize the protrusion (light purple), Rpb2 (partially transparent) has been added and Pol II has been rotated upwards by ~40 degrees compared to panel 1b.
d) TFIIF binding to the PIC. As positioned by site-specific protein cleavage and crosslinking assays [13, 15], dimerization domains of TFIIF subunits Tfg1 (deep purple) and Tfg2 (blue) are located on the Pol II lobe. A C-terminal winged helix (WH) domain in Tfg2 resides on top of the Pol II protrusion domain, close to upstream promoter DNA. In contrast, a cryo EM structure suggested that the human TFIIF WH lies just downstream from the TFIIB core domain (not shown). TFIIB is shown in yellow, TBP in green. EM showed that promoter DNA is slightly bent over the cleft by ~ 18 degrees and the Pol II clamp is in an open state to accommodate dsDNA for the initial step in OC formation [21].
e) A triple WH structure of TFIIE spans the Pol II cleft. As positioned by site-specific protein cleavage assays [16], Tfa1 WH domain (cyan) anchors TFIIE on the Pol II clamp and dimerizes with the Tfa2 tandem WH domain (red) that encircles promoter DNA at positions 18 to 8 relative to the human TSS at +1.