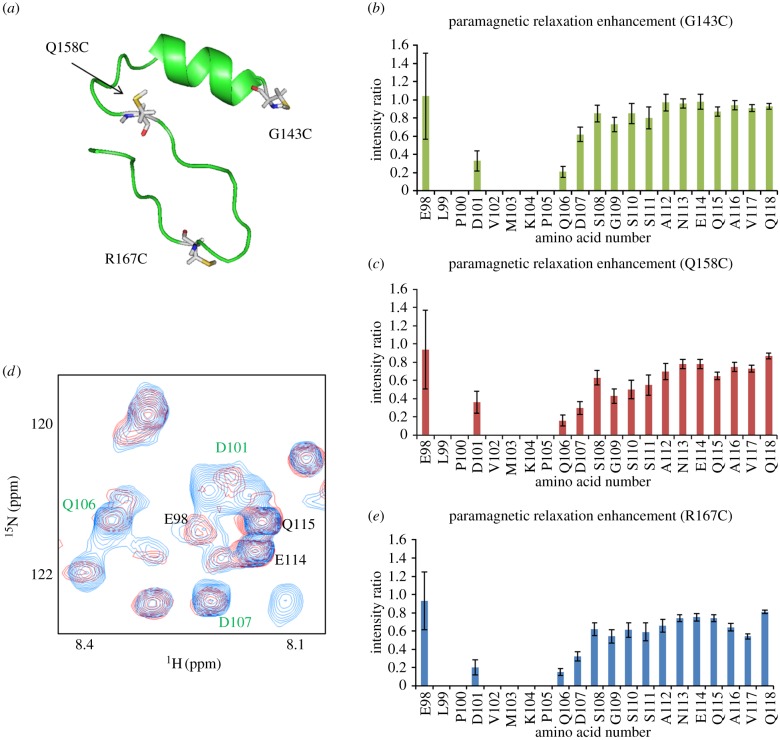
Figure 4.
PRE experiments. (a) The three mutated sites in Vif which were distributed at widely separated positions in the peptide. G143C, Q158C and R167C are highlighted in white and the sulfur of the cysteine to be modified by label is coloured in yellow. Each predicted mutant structure is generated by Pymol in order to determine that the side chain of cysteine would not be buried inside the molecule. (b) Intensity ratio of EloB C-terminus for G143C mutant. (c) Intensity ratio of EloB C-terminus for Q158C mutant. (d) A portion of the 1H-15N HSQC spectra of EloBC in the presence of the paramagnetic molecule-labelled SOCS-box R167C peptide (red) or the diamagnetic molecule-labelled R167C peptide (blue). Peaks assigned to the EloB C-terminal tail are indicated in green (significantly broadened) or in black (not affected). (e) Intensity ratio of EloB C-terminus for R167C mutant. The intensity of peaks from residues 101–104 are broadened owing to the interaction regardless of the paramagnetic effect. The error bars show how the noise of the spectra affects the intensity ratio. Stronger peaks provide smaller deviation.