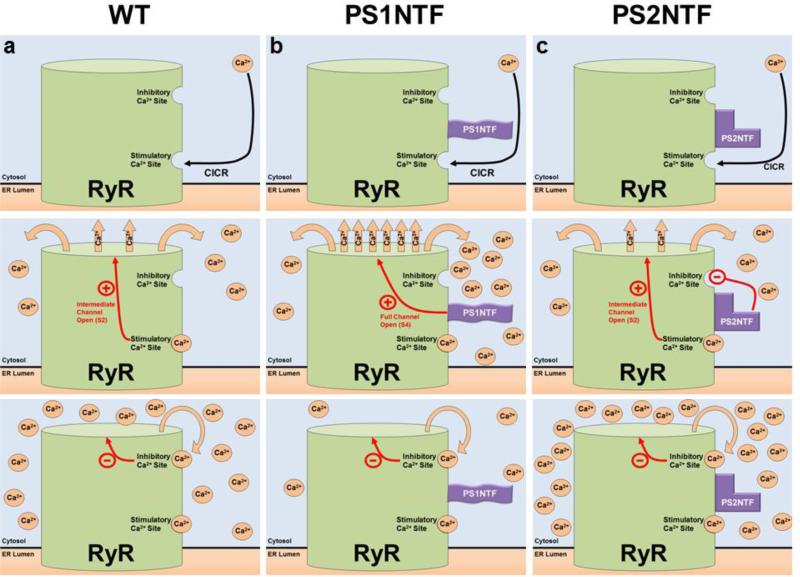
Figure 5. Proposed mechanism of RyR calcium release regulated by the N-termini of PS1 and PS2.
PS1NTF and PS2NTF have distinct effects on RyR calcium induced calcium release (CICR) from ER stores. A) The mechanism of RyR regulation by Ca2+ binding at the high affinity stimulatory site results in moderate channel opening. Ca2+ is released until the local Ca2+ concentration rises to the point at which the low affinity, inhibitory Ca2+ binding site is occupied resulting in closure of the channel. B) The N-terminus of PS1 (PS1NTF) binds RyR and increases calcium release from the ER. Occupation of the RyR high affinity stimulatory site by Ca2+ causes channel gating and Ca2+ release. Bound PS1NTF increases channel opening, causing rapid RyR Ca2+ release until the low affinity inhibitory Ca2+ site is bound, closing the channel and terminating ER Ca2+ release. The increased rate of Ca2+ release due to PS1NTF binding results in overall reduced Ca2+ release because inhibitory concentrations are reached in less time. C) The N-terminus of PS2 (PS2NTF) binds to RyR. Ca2+ ion binding at the high affinity stimulatory site of RyR causes channel gating and Ca2+ release. PS2NTF has no effect on channel gating but blocks Ca2+ inhibition of the RyR channel at high cytosolic calcium concentrations. Significantly elevated cytosolic Ca2+ concentrations result in binding of Ca2+ at the low affinity inhibitory site eventually closing the channel and ending calcium release, however, resulting in an overall higher cytosolic Ca2+ concentration.