Abstract
Trafficking of biological material across membranes is an evolutionary conserved mechanism and is part of any normal cell homeostasis. Such transport is comprised of active, passive, export through microparticles and vesicular transport (exosomes) that collectively maintain proper compartmentalization of important micro and macromolecules. In pathological states, such as cancer, aberrant activity of export machinery results in expulsion of a number of key proteins and microRNAs resulting in their misexpression. Exosome mediated expulsion of intracellular drugs could be another barrier in the proper action of most of the commonly used therapeutics, targeted agents and their intracellular metabolites. Over the last decade, a number of studies have revealed that exosomes cross-talk and/or influence major tumor related pathways such as hypoxia driven EMT, cancer stemness, angiogenesis and metastasis involving many cell types within the tumor microenvironment. Emerging evidence suggest that exosome secreted proteins can also propel fibroblast growth, resulting in Desmoplastic reaction (DR); a major barrier in effective cancer drug delivery. This comprehensive review highlights the advancements in the understanding of the biology of exosomes secretions and the consequence on cancer drug resistance. We propose that the successful combination of cancer treatments to tackle exosome mediated drug resistance requires an interdisciplinary understanding of these cellular exclusion mechanisms, and how secreted biomolecules are involved in cellular cross-talk within the tumor microenvironment.
Keywords: Exosomes, Export Mechanisms, Cancer Drug Resistance, microRNAs
1 Introduction
Aggressive and therapy resistant cancers sustain on robust biological interaction networks arising from gene-gene, gene-microRNA (miRNA), protein-protein, parallel signaling as well as intracellular, intercellular and distant cell interactions [1;2]. The fluidity of such complex biological interaction is maintained by constant influx and efflux of biological material across nuclear and plasma membrane of cells. The advancements in high resolution imaging have revealed that in addition to the well-recognized active [3] and passive transport systems [4], there exists a number of additional transport mechanisms through which cells communicate with the outside environment (within its microenvironment and even at distant sites) [5]. Among them vesicular transport, particularly ‘Exosome’ mediated transport stands out [5]. Over the last three decades, considerable amount of research has done in order to understand the exosome mediated cell-cell communication mechanisms [6]. These efforts have revealed various new facets of material transport across biological membranes and have verified, to a great extent, the role of exosomes in disease development [7;8]. Virtually every type of protein, RNAs [9], breakdown products of signaling pathways, viruses [10] and, as recently discovered, miRNAs [11] can be transported through exosomes. Additionally, as presented in this review, we invoke the concept that anti-cancer drugs (chemo- and targeted agents) may also be subjected to exosomal type of efflux leading to reduced efficacy of different cancer treatment regimens. The consequence of export of such diverse group of entities by exosomes, some known and others yet to be discovered, indicates that much to be learned on the dynamics of vesicular transport.
Cellular transport (nuclear membrane transport, organelle transport and cell membrane transport) is mediated by a number of mechanisms, some of which are active; requiring energy input and a carrier or passive; i.e. through diffusion (from high concentration gradient to lower diffusible entities) [12]. Collectively these transport mechanisms regulate the proper and controlled expression of important biological moieties, ions and especially proteins in the right cellular compartments [13]. Many such transport mechanisms, especially protein transport, activates receptor-mediated signaling through autocrine, paracrine or juxtacrine mechanisms. The active transport is the movement of a moiety across a cell membrane against its concentration gradient [14]. In both eukaryotic and prokaryotic cells, this usually occurs when there is an accumulation of high concentrations of molecules that the cell needs, such as ions, glucose and amino acids. Organelle specific transport plays an important role in the normal cell homeostasis. Particularly, nuclear transport (the movement of moieties in and out of the nucleus) has been very well studied [15]. The entry and exit of molecules >40KDa from the cell nucleus is tightly controlled by the nuclear pore complexes (NPCs) [16]. Although small molecules can enter the nucleus without regulation, macromolecules such as RNA and proteins require association with specialized transport proteins, karyopherins [sub-categorized as importins (that perform import function) [17-20] and exportins (performing export function)] [21]. The proteins that are imported in the nucleus carry a nuclear localization signals (NLS) or proteins without NLS that are in complex with other proteins having NLS, and the transport of such proteins are recognized by importins (mostly importin β family) [22]. Likewise, proteins, transfer RNA, and assembled ribosomal subunits carry a nuclear exclusion signal (NES) that is identified by the specialized exportins, resulting in their export to the cytoplasm [23]. In addition to the above transport mechanisms, researchers have now turned their focus to the recently discovered non-canonical transport mechanisms in nature that involves specialized machinery such as exosomes and microparticles. Here we review some of the current knowledge on these transport mechanisms and how they are playing critical roles in cancer drug resistance.
2 Exosomes
First discovered in maturing mammalian reticulocyte (immature red blood cell), exosomes are ~40 to 100 nm vesicles secreted by a wide range of mammalian cell types [24]. The term exosome (at that time derived from micro-vesicles) was first coined by Trams et al., in the early 1980s [25]. The exosomes consist of a lipid bilayer membrane surrounding a small cytosol and are devoid of cellular organelles. Exosomes contain various molecular constituents of their cell of origin, including proteins and nucleic acid material (all types of RNAs) [26]. Although the exosomal protein composition varies with the cell and tissue of origin, most exosomes contain an evolutionary-conserved common set of protein molecules [27]. The RNA molecules in exosomes include mRNAs, and recently recognized miRNAs. Over the years, researchers have been able to capture exosome biogenesis process in different cell types and have successfully isolated these membranous bodies from diverse sources ranging from blood plasma [28], serum [29], and urine [30].
The biogenesis of exosomes (summarized in Figure 1) is a very tightly regulated process governed by multiple signaling molecules and begins with the receptor activation that is unique for each type of cell [31]. For example, in monocytes and neutrophils, it is the P2X purinoreceptor activation by ATP, in platelets it is the thrombin receptor activation, and for bacterial lipopolysaccharide the toll-like receptor-4 (TLR-4) on dendritic cells have been recognized [32;33]. Morphological studies suggest that exosomes are derived from the micro-vesicle body (MVB) sorting pathway [34;35]. The MVBs first fuse with the plasma membrane and release the vesicles into the extracellular milieu as exosomes. Briefly, the contractile machinery of the cell first draws opposing membranes with the help of soluble NSF attachment protein receptor complex (or simply the SNARE complex) together prior to pinching off the membrane connection and releasing the vesicle into the extracellular space [36-40].
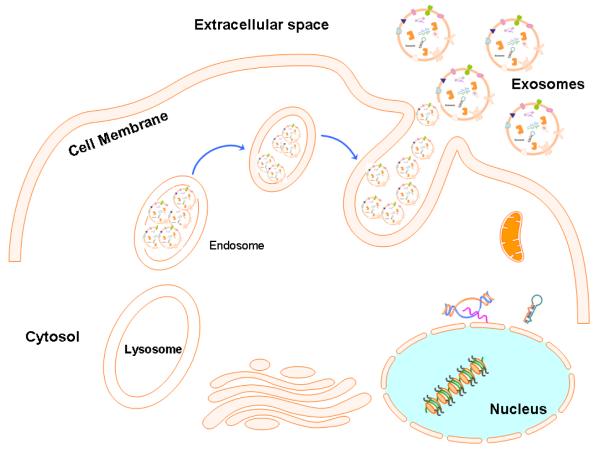
Figure-1. Biogenesis and release of Exosomes.
Diagram depicting the well accepted model for exosome biogenesis and release. Primarily, the exosomes are derived from the multi-vesicular bodies (MVBs) which are known as late endosomes (originally derived from lysosomes). Exosome generation can be triggered by many factor including extracellular stimuli (e.g., microbial attack) and other stresses. The exosomes can be released into the extracellular environment by fusion of MVBs with the cell surface that is supported by a number of specialized proteins called SNAREs.
The release mechanisms of exosomes have been studied in depth as well. Studies from Thery and colleagues demonstrated that a number of Rab family proteins, including Rab27a and Rab27b, act as key regulators of the exosome secretion pathway [41]. Rab27 has been shown to be involved in cancer progression and tumor promotion, which provided early indications that components of exosome secretion pathway may have roles in tumor biology [42]. Apart from Rab27a and b, another Rab family member protein, Rab35 has been shown to regulate exosome secretion by interacting with GTPase-activating protein TBC1 domain family, member 10A-C (TBC1D10A-C) [43]. Exosome release has been shown to be triggered by various other stimuli as well including ceramide [44] and changes in membrane pH [45]. Studies have also shown that exosomes can be released into the extracellular mileu by the outward exosome and micro-vesicle budding pathway (EMV) in which budding/fission of the plasma membrane occurs [46]. The regulation of EMV pathway is multifactorial and is sensitive to intracellular calcium levels is dependent on the cell’s structural scaffolding. The summary diagram of the most acceptable model of exosome formation and release of exosomes is in Figure 1.
3 Exosome Structure
High resolution microscopy coupled with advanced proteomic analyses of exosomes from different disease models has provided deeper insights into the structural composition of exosomes (summarized in Figure 2). Over the years, it has been recognized that not only the contents inside the exosomes but also the components in exosome structure can influence distant cell signaling. A number of important reviews have elaborated on building blocks of exosomes, and some of the main salient features are described below:
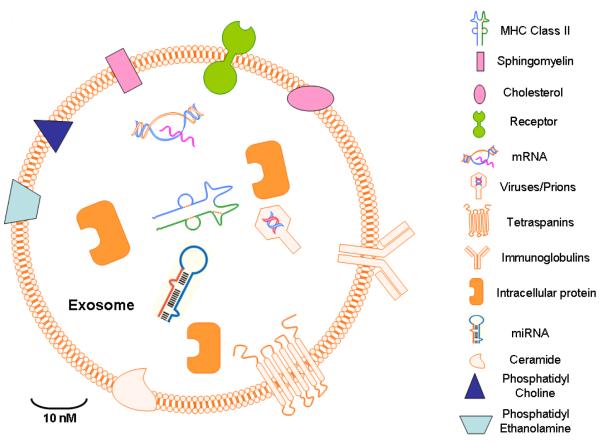
Figure-2. Exosome Structure.
Exosomes are capable of hosting a wide array of molecules including viruses, nucleic acid material (RNAs, DNA), and microRNAs depending on a variety of factors including the cell type or origin. Other factors influencing exosome content include pathological state of the host organism. The contents of exosomes can be transferred from the cell of origin to their target cells in local microenvironment or even at distant site that can possibly give rise to an exponential intercellular communication networks.
Primarily, exosomes carry proteins membrane transport and fusion proteins such as Rab GTPases, annexins, flotillins, genes and proteins for micro-vesicle body biogenesis proteins such as Alix and tumor susceptibility gene Tsg101 [47]. Additionally, the protein families that are associated with lipid microdomains, such as integrins and tetraspanins such as CD9, CD81, CD82, CD83 and CD63 are also important part on the regulatory role of exosome [48]. The pool of exosome proteins are both conserved and also cell type specific i.e. depending on the cells from where exosomes are secreted. Heat shock proteins (Hsp), CD63 and tetraspanins are the most prominent evolutionary conserved proteins in exosomes [49]. Cytoskeleton and metabolism related proteins form the pool of other frequently found proteins that include β-actin, tubulins, myosin, cofilin glyceraldehyde 3-phosphate dehydrogenase and major histocompatibility complex (MHC) class I and II molecules [50]. Of interest, exosomes also contain proteins that are involved in cell signaling pathways, like Wnt-β-catenin signaling proteins [51;52], the Notch ligand Delta-like 4 [53] along with proteins involved in intercellular cell signaling, such as interleukins [54]. The role of these multi-faceted proteins in cancer development, sustenance and drug resistance is further elaborated in later sections of this article.
The lipid content of exosomes can be categorized into two types (a) either conserved or (b) matching the characteristics of the cell from where they are originating [55]. Exosome lipidomics have helped in sorting of these lipids in different model systems [56]. These structural lipids do not only give shape to exosomes but have also shown to take part in cell communication by modulating the pathways in cells far away from their site of origin. For example, the lipid content in exosome structure or the ones excluded by them have been shown to suppress critical cancer survival pathways such as Notch leading to cancer cell death in a pancreatic model [57]. Extensive evaluation from different cell types such as hematopoietic cells, cancer cells, internal micro-vesicle bodies (MVBs) etc. have shown that exosomes carry lipids that are enriched in lysobisphosphatidic acid; a key moiety in exosome biogenesis pathway that interacts strongly with another exosome biogenesis Alix [58]. Collectively, lysobisphosphatic acid and Alix induce the formation of internal MVBs from liposomes through internal budding. Exosomes are enriched in lipids that are associated with lipid rafts, e.g sphingolipids, cholesterol, glycerophospholipids and ceramide that have characteristics elongated saturated fatty-acyl chains [59]. Signaling mediators including prostaglandins, arachidonic acid, phospholipase A2, phospholipase C and D, etc. are also component of exosome lipid pool [60;61]. Collectively, the structure of exosomes is recognized to carry a variety of important proteins and cohesively they interact guiding the intercellular communications in normal and disease states.
Exosomes are secreted by a variety of cells types and their contents are recognized to play critical roles in inter- and intracellular communications for diverse cell types [62]. The ExoCarta database (http://exocarta.org/, a database for exosome target identification and function) lists 4563 proteins, 1639 mRNAs and 764 miRNAs as export targets of exosomes collected from 146 published studies on the web [63;64]. These numbers reflect the enormity of targets that can be modulated by exosomes, and further highlight the importance of studying them in the context of cancer. For example, maturing reticulocytes release obsolete membrane proteins such as the transferrin receptor by means of exosomes [65]. Activated platelets release exosomes with multiple warheads including prions affecting distant cells (as discussed in later sections of this review) [66]. Exosomes are also secreted by cytotoxic T cells, and serve as efficient resource for cytolytic substances for target cells [67]. While, antigen presenting cells, such as B lymphocytes and dendritic cells (DC) secrete MHC class-I- and class-II-carrying exosomes that stimulate T cell proliferation in vitro [68]. The fact that exosomes can secrete such a wide variety of different proteins, most of which have recognized roles in influencing multiple signaling pathways, which prompted in-depth studies on exosomes in cell-to-cell signaling. Researchers have hypothesized that exosomes can regulate the function of distant cells by releasing their contents far away from the site of origin and may influence processes in the recipient cells, especially promoting interaction between multiple cell types within the tumor microenvironment. For example, RNA that is shuttled from one cell to another, known as “exosomal shuttle RNA,” could potentially affect protein production in the recipient cell [69]. By transferring molecules from one cell to another, exosomes from certain cells of the immune system, such as dendritic cells (DC) and B cells, may play a functional role in mediating adaptive immune responses to pathogens and tumors [70]. Conversely, exosome production and content may be influenced by molecular signals received by the cell of origin [71]. Microparticles are another form of exporters that are small membrane bound vesicles circulating in the blood derived from cells that are in contact with the bloodstream such as platelets and endothelial cells [72]. Because they retain the signature membrane protein composition of the parent cell, they have been recognized to influence cell behavior even at distant sites and play key role in disease pathology [73;74]. Nevertheless, in this review, we focus on exosomes only and not on many other secretory vesicles or micro-particles.
4 Exosome Secreted Proteins and its Consequence in Cancer
There is a consensus that exosomes guide the export of major types of proteins and transcription factors to the outer-cellular milieu [75]. Depending on the context, these proteins are either tumor promoters or tumor suppressors. Such exosomal secretion of proteins is expected to impact distant cell signaling or promote a niche that sustains tumor microenvironment leading to disease spread. Each week, a new protein is added among the exosome cargo export list (data obtained from Pubmed search). While new exosome target proteins are constantly being discovered, here we highlight some of the major proteins that are known to be secreted by exosomes and summarized the consequence of their export in terms of de novo and acquired cancer resistance.
4.1 HSP
Heat Shock proteins (hsps) are a class of functionally related proteins that are activated in response to high temperature and other stresses [76;77]. They have been studied for their roles in stress response, as chaperons proteins, housekeeping genes, cardiovascular function and antigen presentation [78]. Hsps have been used as immunologic adjuvants to boost the response to a vaccine and for also increasing the efficacy of a vaccine [79;80]. Mathew et al., discovered the presence of Hsp-70 in exosome from mammalian and avian reticulocytes as well as from a differentiating avian erythroleukaemic cell line [81]. Their studies also revealed a close association of Hsp-70 with the transferrin receptor (TFR), a protein lost during reticulocyte maturation leading to the assumption that Hsp-70 plays a key role in exosome formation and/or release in immature red cells. Lancaster and colleagues demonstrated that exosomes contribute to the release of Hsp70 from human peripheral blood mononuclear cells (PBMCs) in both basal and heat stress-induced states via a lipid raft-dependent pathway [82]. These studied cemented the foundations of a novel hsp secretory pathway in both the basal and stress-induced state. While investigating the effect of cellular stress on the exosomes produced by B-lymphoblastoid cell lines Clayton et al., observed a differential expression of hsps and other exosome markers such as MHC class I, CD81, and LAMP-2 [83]. Interestingly in their system, the exosomes from control or heat-stressed B cells did not trigger dendritic cell maturation. Direct evidence on the influence of hsp release by exosomes in cancer came from Hong-Li’s group showing that anticancer drugs cause release of exosomes with hsps from human hepatocellular carcinoma cells that otherwise have a defective cytotoxic pathway that elicit effective natural killer cell anti-tumor responses in vitro [84]. Additionally, Cho and colleagues demonstrated that hsp enriched exosomes can elicit anti-tumor response in murine model in a MHC-independent manner [85]. These are some of the examples where exosome secretion of hsp can be harnessed as therapeutics in cancer.
4.2 P53
Considered the guardian of genome, p53 is a central player regulating many different cell surveillance pathways [86]. Its critical role in normal cell physiology is highlighted by the fact that in >50% of tumors, p53 is found either mutated or lost [87]. In tumors, where p53 is functional, it is under tight regulation by the negative regulator MDM2 that promotes its ubiquitin dependent degradation [88]. Therefore, pharmaceutical re-activation of p53 pathways is being pursued as a major form of cancer therapy [89]. Nevertheless, there are other epigenetic regulatory control mechanisms including the regulation by miRNAs and exosomes mediated exclusion regulation that renders p53 re-activating pharmaceutical strategy ineffective. In a proteomic study from Levine’s group, the exosomes production of p53 was investigated [90]. The authors demonstrated that p53 promotes exosomes production leading to secretion of numerous p53 target cells in the extracellular environment. This in turn appears to have major implications in adjacent cell communication and immune activation. The same group investigated p53 in endosomal compartments as well [91]. Their studies proved that p53 regulates transcription of the numerous important genes such as TSAP6 and CHMP4C, which enhance exosome production. Simultaneously, p53 could also enhance the expression of CAV1 and CHMP4C; genes for endosomal clearance of the EGFR receptor from the plasma membrane. This mechanism has shown to retard cell growth and division along with cutting down the cells’ capability to utilize catabolic resources post-stress signal. Conversely, Lespagonol and colleagues highlighted that suppression of TSAP6 in a mice model severely impairs p53 mediated production of exosomes [92]. P53 mediated exosomes production was also shown to directly influence human papillomavirus (HPV) E6/E7 content in vesicles in an HPV carcinoma model [93]. These and other studies have provided considerable insights into the cross-talks between endosomes, exosomes and transcriptional signaling mediated by p53 [94]. However, much more needs to be learned on the role of exosomes on other family members in the p53 pathway such as p63 and p73 that share similar functions. Collectively, these findings promote the concept that exosomal regulation of p53 and its targets need to be considered in the design of any therapeutic strategy against this master transcriptional regulator.
4.3 PTEN
Phosphatase and tensin homolog (PTEN) is a protein that, in humans, is encoded by the PTEN gene and acts as a tumor suppressor [95]. It is ranked among the top most frequently mutated genes in all major cancers [96]. Primarily, PTEN works by keeping PI3K-AKT pathway (found constitutively activated in cancers) in check and regulates cell cycle progression [97]. Putz and colleagues showed that PTEN, which is normally localized in nucleus or cytoplasm of a cell, was secreted in exosomes and showed phosphatase activity in target cells, resulting in the suppression of cell proliferation [98]. These findings have been corroborated by independent groups confirming that the exosomal transfer of PTEN and its consequence on affecting target cells is an important biological phenomenon [99]. In a pancreatic model, studies have shown that exosomes mediate the suppression of hairy and enhancer-of-split homolog-1 (Hes-1), the intra-nuclear target of Notch-1 signaling pathway, resulting in the activation of apoptotic cascade after a cell cycle arrest in G0/G1 phase and re-expression of PTEN [100]. These findings highlight the reciprocal regulations between Notch signaling and PTEN/GSK-3beta, leading to the conclusion that the interactions of exosomes target cells, hampers the functioning of the Notch-1 survival pathway in a PTEN dependent manner. In summary, these findings open newer avenues for investigating the effects of PTEN activation not only in the cell of origin but also in distant cells where they are exerting their functions.
4.4 APC
Adenomatous polyposis coli (APC) is a tumor suppressor protein encoded by APC gene and mutations in this gene has been shown to contribute in the development of colorectal cancer (CRC) [101]. Primarily, APC is known to regulate β-catenin function (a protein required for the regulation of Wnt signaling) [102]. While studying for the presence of APC in exosomes, Lim and colleagues performed comparative proteomic profiling of exosomes from SW480 and SW480APC (stable transfected with full length APC) colon cells [103]. Their analysis revealed contrasting functions of APC in either model. In the exosomes from APC transfected cell line SW480APC, there was an enhanced expression of Wnt antagonist Dickkopf-related protein 4 (DKK4) that was not found in parental SW480. These findings indicate that in the context of cancer, exosome-mediated secretion of DKK4 may be a mechanism used by epithelial colon cells to regulate Wnt signaling, which is typically lost during CRC progression. Such findings provide indications on the extracellular regulatory roles of APC that in principle can be targeted using exosomes.
5 MicroRNA Export by Exosomes
MicroRNAs (miRNAs) are small non-coding RNAs with diverse functions [104]. They are recognized to regulate a number of genes by weakly binding to the 3’UTR of their target mRNA, resulting in the deregulation of their target gene expression [105]. A single miRNA can influence multiple genes within a single cell or can even effect the expression of genes in adjacent cells within the microenvironment. The distant cell influence of miRNAs certainly has an element of export mechanism attached to it. Supporting this observation, miRNAs have been observed both at their primary site (i.e. inside of a cell) as well as in the circulation [106]. The circulatory miRNAs are being intensively investigated as biomarkers in different cancers [107]. Traditionally, miRNA export has been well recognized to be mediated through active transport by specialized nuclear exporter XPO5 [108]. Interstingly, recent studies suggest that the secretion of miRNAs is also in part mediated through vesicular/exosome mediated mechanisms [109]. A recent study showed that most of the miRNAs detected in human saliva were concentrated in exosomes [110]. These findings point to the fact the exosomes have a major part in circulatory miRNA biology. To help understand circulatory miRNAs and their function, databases such as miRandola were developed [111]. The miRandola is a database containing >2132 entries, with 581 unique mature miRNAs information derived from 21 types of samples. In this database the miRNAs are classified into four categories, based on their extracellular form: miRNA-Ago2 (173 entries), miRNA-exosome (856 entries), miRNA-HDL (20 entries) and miRNA-circulating (1083 entries). The miRandola is available online at: http://atlas.dmi.unict.it/mirandola/index.html. Tools such as Exocarta discussed above and miRandola serve as starting point for circulatory miRNA research hypotheses development and provide excellent resource for the study of exosome related miRNA export function in different diseases especially cancer.
The role of exosomal miRNAs in regulating intercellular communications has been established [112]. Valadi et al., were the first to show exosome mediated miRNA transfer and promoted the idea that this could be a mechanism of genetic exchange between cells [113]. In their experiments, the transfer of mouse exosomal RNA to human mast cells resulted in the expression of mouse proteins in humans [114]. Later on, Gibbins group showed that exosomal transport can modulate the activity of miRNA as well [11]. Following these initial discoveries, Koga and colleagues demonstrated that encapsulation by exosomes can protect miRNAs in colon cancer cell line culture supernatant in an extreme condition model (fecal matter) [115]. The study proved that miRNA integrity is maintained in an exosomal cushion indicating that they can remain functional when transferred to distant sites outside the cell of origin. Chen and colleagues have suggested that miRNAs in exosomes secreted by mesenchymal stem cells (MSC) are predominantly pre- and not mature miRNAs [116]. In this system, RNA-induced silencing complex-associated proteins were not observed, suggesting that only the pre-miRNAs but not the mature miRNAs in MSC exosomes have the potential to be biologically active in the recipient cells. However, later on both pre and mature miRNAs were discovered in exosomes in multiple types of cells and tumor models. In addition to miRNA export, Flynt and colleagues showed that exosomes can also induce miRNA splicing in a Drosophila model [117]. Later on, a number of groups proposed the use of exosomal miRNAs for diagnostic and prognostic purposes in lung [118], glioblastoma [119], esophageal squamous cell carcinoma [120], prostate [121;122], ovarian [123], breast [124], colon [125], kidney [126], leukemia [127] and other types of cancer [50]. One of the mechanisms preventing miRNA decay is its loading into the RNA-induced silencing complexes (RISC). Recent work showed that RNA granules in the cytoplasm that are rich in RISC complexes are in close association with endosomes. In addition to miRNAs, certain components of the RISC (GW182 and Argonaut proteins) are also present in exosomes. These findings solidified the concept that, miRNA function, and exosome-mediated intercellular communication converge.
Having verified that miRNA can be exported through exosomes, researchers shifted their attention to investigate the consequence of such export in biological systems especially tumors. Exosome secreted miRNAs have been shown to induce a number of biological functions including modulation of immune response as recently reviewed by Bobrie et al [128]. Middledrop’s group demonstrated that human Epstein Barr virus (EBV) derived miRNAs can be delivered via exosomes and regulate target genes in the recipient non-viral cells [129]. Conversely, it was shown that malignant transformation alters certain pathways which, in turn, changes exosome released miRNAs, which suggest that malignant transformation alters the pathways mediated through specific miRNAs that are exported from cells [130]. In relation to tumors, in a breast model, Yang and colleagues showed that exosome secreted miRNAs enhance the invasive potential of breast cancer cell lines [131]. In a recent study, the exosome transported miRNAs were shown to induce leukemia cell to endothelial communication signifying their role in promoting interactions between distinct population of cells and also confer pro-angiogenic effects [127]. Furthermore, Bullerdiek et al demonstrated that exosome mediated delivery of miRNA of chromosome 19 miRNA cluster (19q13 is recognized as a non-random cytogenetic abnormality in thyroid adenomas) act as immune modulators in pregnancy and tumorigenesis [132]. Collectively, these studies show that exosomes play an important role in miRNA regulation and one cannot ignore them while studying miRNA targets or in designing miRNA targeted therapeutic strategies.
6 Exosomes in Cancer Resistance
De novo and acquired resistance to chemo, radiation and targeted therapies remains a major stumbling block in cancer treatment [133]. The development of resistance is multi-factorial that includes switching of cancer cells to secondary salvage pathways when the primary hallmark is shut [134], epigenetic suppression of tumor suppressor protein activation by miRNAs [135], presence of a sub-population of highly resistant cancer stem like cells with enhanced plasticity such as epithelial-to-mesenchymal transition (EMT) phenotype [136], low drug penetrance (due to Desmoplastic reaction or DR in the tumor microenvironment) and others [137]. The role of exosomes in modulating the above described pathways that leads to the development of cancer radio- and drug-resistance is an emerging area of intense research (Figure 3). For example, Khan and colleagues have shown that exosomal secretion of survivin suppresses the efficacy of proton irradiation in a cellular model [138]. In this study, it was shown that even though the rate of exosomal secretion was not increased by proton radiation, yet the surviving content within the secreted exosomes was higher which, in turn, promoted cell proliferation and metastatic potential. In another study, the role of exosomal secretion in complement resistance in cancer was established [139]. While studying the membrane damage inducer complement system/membrane attack complex (MAC), the authors showed that as a preventive mechanism cells release exosomes with abundance of protein Mortalin and complement MAC that prevents membrane lysis by the complement system [140]. In another study, Zhang and colleagues demonstrated that membrane form of TNF-alpha secreted in exosomes prevents cytotoxic T cell activation-induced cell death [141]. This resulted in suppressed activity of commonly used pore formers such as streptolysis O and melittin. Bodey et al, demonstrated that in mammalian ontogenesis, the thymic endodermal epithelial anlage develops and differentiates into a complex cellular microenvironment that is regulated by dendritic exosomes [142]. These dendritic type exosomes within the mammalian thymic microenvironment were projected to play a role in antigen presentation in the dendritic neuro-endocrine-immune cellular network. In another study, lymphoma exosomes were shown to shield target cells from antibody attack by releasing CD20 [143]. The biogenesis of exosomes in this setting was found to be modulated by the lysosome-related organelle-associated ATP-binding cassette (ABC) transporter A3 (ABCA3) which is recognized to cause resistance to chemotherapy [144]. Similarly, exosomes influence on the development of cisplatin resistance has been proven in a ovarian cancer model [145]. In this study, using a cisplatin resistant and sensitive paired ovarian cell line, it was shown that in the resistant setting there was statistically significant reduction of cisplatin in the lysosomal compartment. The minimal amount of stored cisplatin was rapidly excluded from the lysosomes aided by the enhanced expression of cisplatin transporters. In another investigation, Jin et al detected enhanced secretion of Annexin 3 protein in vesicles from cisplatin resistant ovarian cancer cell lines [146]. The number of exosomes in these ovarian cell lines was directly correlated with the expression status of Annexin 3 protein as well. These findings point to the role of certain proteins in promoting exosomes secretion as well. Similarly, in a breast Her-2 expressing cell line model, the resistance to Trastuzumab was linked to the secretion of Her-2 over-expressing exosomes [147]. These authors found that the release of exosomes was modulated by EGF and heregulin that are recognized as Her-2 receptor activating ligands. Their cell line findings could be replicated in Her-2 positive tumor cell-conditioned supernatants or in breast cancer patients’ serum bound to Trastuzumab. These investigations conclusively linked Her2-positive exosomes in modulating sensitivity to Trastuzumab, and, also to Her2-driven tumor aggressiveness.
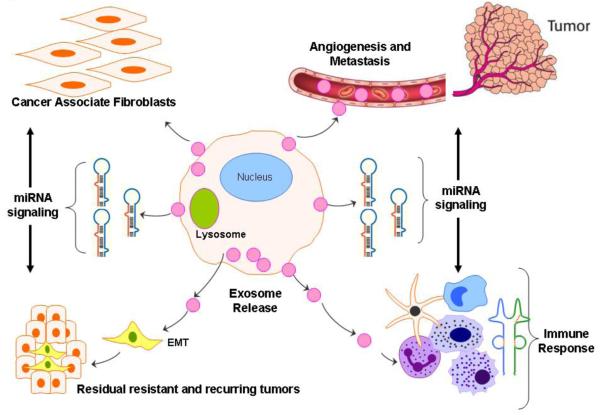
Figure-3. Role of Exosomes in Sustaining Cancer Resistance Networks.
Exosome mediated export of biological material can induce a microenvironment favorable for resistance. Exosome released factor can promote (a) EMT cell morphology, resulting in stemness; (b) promote fibroblast like cell formation that causes desmoplatic reaction (stromal reaction); (c) promote immune escape mechanisms and (d) promote angiogenesis and metastasis. The miRNAs expelled by exosomes can regulate multiple signaling pathways that cumulatively promote resistant phenotype of most tumors.
The above important findings rushed in an era of large scale proteomic analysis of tumor derived exosomes that provided much of the insights on resistance signatures in different cancer models. These studies provided deeper insights on the content of secreted exosomes and also shed light on how exosomal components exert their function supporting their clinical application in immunotherapy regimens. For example, proteomic profiling of exosomes was performed on prostate cancer cell lines with distinct AR phenotypes [148]. In this study, a comprehensive proteomic and lipidomic analysis of prostate derived exosomes was performed to identify biomarker and therapeutic targets for the treatment of prostate cancer. Similarly, other researchers have performed proteomic analysis on exosomes derived from different cancer cell types and biological fluids such as exosomes from human pleural effusion [149], human mesothelioma cells [150] and exosomes derived from melanoma cells [151]. In the following sections, we will elaborate how exosomal secretions influence the major signaling networks that are recognized to play pivotal role in the sustenance of cancer, and how they influence de novo and acquired drug resistance.
7 The role of Exosomes in tumor microenvironment, cancer metastasis and metastatic niche
Understanding the complexity of the tumor microenvironment is daunting. A number of elements are involved in seeding and maintenance of the tumor microenvironment including cells from different lineages, soluble factors, signaling molecules, extracellular matrix, hypoxia and mechanical cues (as discussed here in the form of exosomes) that could promote neoplastic transformation. These combinations of factors support tumor growth and invasion by protecting the tumor cells from host immunity and fostering therapeutic resistance, and provide niches for metastases to thrive. The metastatic niche receives constant inputs from the above mentioned cues that educate tumor cells to become aggressive and therapy resistant. Such tumor cell education within the microenvironment is being intensively investigated and has been suggested to be through various mechanisms including a) cell-cell and cell-matrix interactions; b) local release of soluble factors promoting survival and tumor growth (crosstalk between stromal and tumor cells); c) direct cell-cell interactions with tumor cells i.e. trogocytosis; d) generation of specific niches within the tumor microenvironment that facilitate the acquisition of drug resistance; or e) conversion of the cancer cells to cancer-initiating cells or cancer stem like cells. Collective analysis of each of these factors points to some form of communication between different components of the microenvironment as the underlying/encompassing principle and in essence gives substance to our ‘exosome signaling hypothesis’. In the following paragraphs, we will individually discuss the published studies that have proven the roles of exosome mediated transport in diverse mechanisms such as metastasis and angiogenesis, hypoxia, EMT signaling, TGF-β signaling, Wnt-β-catenin signaling that collectively support the tumor microenvironment niche.
7.1 Exosome in Metastasis
Most cancer deaths are the result of metastasis which in is generally refractory to any form of available therapies [152]. Researchers are still grappling to understand the underlying drivers of migratory cells and their ability to escape the site of origin in primary tumors [153]. Exosomes are known mobile elements that function as escape routes for proteins and miRNAs (some of which can be promoters of metastatic pathways) from one cell (site of origin) to distant locations. As expected, the role of exosome mediated signaling in cancer metastasis is also emerging. For example, Grange and colleagues showed that exosomes released from renal cells can promote angiogenesis in lung cancer ascites [154]. The breakthrough in the understanding of exosome’s interaction with endothelial cells, angiogenesis and metastasis promoters came with the advent of fluorescent exosome labeling techniques. Using these techniques, melanoma exosome was observed to interact with and influence endothelial cell morphology through exosomes [155]. This was concurrent with the production of endothelial spheroids and endothelial sprouting. Similarly, exosome mediated release of melanoma cells was shown to prime sentinel lymph node metastasis [156]. These are examples where tumor exosomes elicited paracrine endothelial signaling thereby contributing to metastasis spread of the tumors. Recently, Rana and colleagues investigated how tumor derived exosomes educate selected host tissues towards pro-metastatic phenotype [157]. In this study, tumor exosomes were shown to target non-transformed cells in pre-metastatic organs and modulate pre-metastatic organ cells predominantly through transferred miRNAs. The exosome delivered miRNAs were found to mostly target metastasis related pathways such as proteases, adhesion molecules, chemokine ligands, cell cycle- and angiogenesis-promoting genes, and genes engaged in oxidative stress response. In a prostate model, large oncosomes (oncogenic exosomes) containing metalloproteinases, RNA, caveolin-1, and the GTPase ADP-ribosylation factor 6, and factors that are active toward tumor cells, endothelial cells, and fibroblasts were shown to be present in the circulation of mice carrying metastatic disease [158]. These are prime examples where migrating tumor cells and exosomes condition the tumor microenvironment and distant sites, thereby potentiating disease progression.
7.2 Exosomes, Hypoxia and Tumor microenvironment
Hypoxia is an emerging area that is a critical component promoting the sustenance and spread of epithelial tumors [159]. The seeding of tumor stroma [Desmoplastic reaction (DR)], a barrier to efficient drug delivery, has been clearly linked to be supported by hypoxia and related pathways [160]. The hypoxic environment (a niche within tumor) is recognized to harbor cells that are drug resistant (compared to the bulk of the tissue) carrying markers that are reminiscent of epithelial-to-mesenchymal transition (a hall mark of cancer stem like cells as discussed in detail later) [161]. It was recently documented that hypoxia promotes the secretion of various tumor promoting factors that influence adjacent tissues in the tumor microenvironment [162]. Therefore, either directly targeting hypoxia or the factors promoting this important phenomenon is an emerging form of therapy under investigation [163;164]. A number of studies have indicated that hypoxia promotes exosomes secretion in different tumor types. In a breast model, King and colleagues showed that hypoxia mediated activation of HIF-1α enhances the release of exosomes and results in aggressive cell phenotype [165]. Concurring with these findings, very recently, exosomes were shown to reflect the hypoxic status of glioma cells [166]. In this model, exosome secretion was found to be promoted in a hypoxia dependent manner leading to the activation of vascular cells during tumor formation. While in a kidney tumor model, Borges and colleagues showed that TGF-β1 (a promoter of EMT)-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative response and fibrosis [167]. The fibroblast activation was found to be dependent on the success of exosomes to primarily deliver TGF-β1 mRNA to the site of fibrosis. These findings certainly have implications in designing an exosome-based therapeutic strategy for targeting fibrosis. Another important study by Park et al., showed that cells in hypoxic microenvironment promote angiogenic pathways and increased metastatic potential through exosomal secretion of certain specialized proteins tetraspanins and Alix in a A431 carcinoma cell line model that was subjected to hypoxia and re-oxygenation [168]. Similarly, in a highly malignant glioma system, hypoxia was shown to promote pro-angiogenic pathways through cancer associated vesicle (exosomes) secretion [169]. The angiogenesis promoted by exosomes was mediated through up-regulation of protease-activated receptor 2 (PAR-2) in epithelial cells. These findings led the same group to develop the hypothesis that the presence of exosomes reflects the hypoxic status of aggressive glioma cells [170]. Tumor cells exposed to hypoxia secrete exosomes with enhanced angiogenic and metastatic potential, suggesting that tumor cells adapt to a hypoxic microenvironment by secreting exosomes to stimulate angiogenesis or facilitate metastasis to more favorable environment [171]. These and other studies verify the role of hypoxia in promoting exosomes secretion that in turn creates a microenvironment favorable for pro-angiogenic environment leading to the development of aggressive tumors.
7.3 Exosomes in Epithelial-to-Mesenchymal Transition
Epithelial-to-mesenchymal transition (EMT), driven by a complex interaction network [172] is considered one of the hallmarks of aggressive tumors [173]. Cells undergoing EMT have enhanced plasticity and propensity to migrate out from the site of origin, resulting in tumor spread [116]. The EMT type cells have been recognized to secrete factors that affect adjacent cells and tissues and contribute to resistance by maintaining the overall tumor microenvironment [174]. The role of exosomes in EMT promotion has been delineated recently. Garnier and colleagues have demonstrated that cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor [175]. Similarly, Roccaro et al., showed that exosomes released from bone marrow derived mesenchymal cells (BM-EMT cells) can promote multiple myeloma (MM) formation in an animal model system [176]. In this study, MM promotion was shown to be governed by exosomal miR-15a highlighting the role of mobile miRNAs in distant cell communication especially in the regulation of EMT related pathways.
7.4 Exosomes, TGF-beta signaling and Tumor Stroma
TGF-beta has for long been recognized to play an important role in the promotion and maintenance of tumor stroma as well as in the induction of EMT [177;178] and is used as a model to artificially induce EMT in cell culture models [179]. While studying the effect of tumor cell-derived exosomes on mesenchymal stromal cells by treating adipose tissue-derived MSCs (ADSCs) with breast cancer-derived exosomes, Cho and colleagues showed that these exosome-treated ADSCs exhibited the phenotypes of tumor-associated myofibroblasts (TAM) with increased expression of α-SMA (a promoter of DR) [180]. The TAM formation by exosome treatment was concurrent with an increase in tumor-promoting factors SDF-1, VEGF, CCL5 and TGF-beta. Collectively, the results show that tumor-derived exosomes induced the myofibroblastic phenotype and functionality of ADSCs via the SMAD-mediated signaling pathway indicating that their contribution to the progression and malignancy of tumor cells is indeed mediated by converting MSCs within tumor stroma into tumor-associated myofibroblasts in the tumor microenvironment. Analyzing the role of exosomal export of TGF-beta, Clayton and colleagues showed that exosomes rich in TGF-beta can suppress the lymphocytes response to interleukin 2 [181]. Similarly, the family member TGF-alpha was shown to stimulate the secretion of exosomal hsps, resulting in the enhancement of human skin cell migration during wound healing in a skin plasticity model [182]. In another study, Clayton and colleagues showed that NKG2D ligand act as a physiological target for exosome-mediated immune evasion in cancer [183]. Here, the authors demonstrated that exosomes derived from cancer cells express ligands for NKG2D and express TGF-beta1, and they further investigated the impact of such exosomes on CD8(+) T and NK cell NKG2D expression and on NKG2D-dependent functions. In another study, Cho and colleagues determined the biological effect of exosomes from two ovarian cancer cell lines (SK-OV-3 and OVCAR-3) on adipose tissue-derived MSCs (ADSCs) [184]. They reported that exosome treatment induced ADSCs to exhibit the typical characteristics of tumor-associated myofibroblasts, with increased expression of alpha-SMA, and also increased expression of tumor-promoting factors such as SDF-1 and TGF-beta. This phenomenon was correlated with an increased expression of TGF-beta receptors I and II. In essence, the exosomes from ovarian cancer cells induced the myofibroblastic phenotype and functionality in ADSCs by activating an intracellular signaling pathway, suggesting that ovarian cancer-derived exosomes contribute to the generation of tumor-associated myofibroblasts from MSCs in the tumor stroma.
7.5 Exosomes in Wnt-β-catenin signaling
Wnt signaling plays central role in tissue development, and aberrations in this evolutionary conserved pathway has been shown to be linked with the development of cancers [185]. Targeting of Wnt signaling for cancer therapy has been intensely investigated [186]. A major component in Wnt signaling pathway is the β-catenin protein that acts as an intracellular signal transducer and functions as a dual function protein, regulating the coordination of cell–cell adhesion and gene transcription, processes that are essential for early embryonic development [187]. Increased nuclear -catenin has been shown in various cancers [188]. Nevertheless, the underlying mechanism on how Wnt controls intercellular interactions and transports between cells remains elusive. In a seminal study Korkut et al., showed that Wnt is transported between cells through exosome-like vesicles containing the Wnt-binding protein Evenness Interrupted/Wntless/Sprinter (Evi/Wls/Srt) [189]. This was the first investigation showing exosomal Wnt transport between cells. The same group further investigated the mechanism of transport in greater detail by using RNA silencing assay to screen for critical genes that prevent the release of Evi vesicles. In this study the authors identified two proteins, Rab11 and Syntaxin 1A (Syx1A), that were required for Evi vesicle release. Interestingly, in an in vivo neuromuscular junction, Syx1A, Rab11, and its effector Myosin5 were required for proper Evi vesicle that show conservation with exosomes in other systems as well [190].
Exosome mediated regulation of β-catenin signaling has also been studied. Two tetraspanins (abundantly found in exosomes) CD82 and CD9 were shown to strongly suppress β-catenin-mediated Wnt signaling activity and led to a significant decrease in β-catenin protein levels [191]. These findings pointed to the role of proteins (found enriched in exosome) in mediating the down-regulation of Wnt signaling through exosome mediated discharge of β-catenin. Another study demonstrated that exosomes derived from plasma cells carried high expression of Frizzled receptor (component of Wnt signaling) and that could suppress the proliferation of activated CD4(+) T by inducing apoptosis through regulation of Wnt signaling [192].
While the above studies proves that exosome mediated regulation of Wnt signaling is important, the reverse effects have also been observed (i.e. Wnt signaling molecules regulating exosome secretion). Cooper and colleagues showed inducible release of exosomes in primary cultured rat microglia following treatment with recombinant carrier-free Wnt3a; an oncogenic protein [193]. Interestingly proteomic profiling of these microglial-derived exosomes carried proteins involved in cellular architecture, metabolism, and protein synthesis and their degradation. These findings have established that Wnt signaling either induces exosome secretion or the components in the Wnt signaling pathway that can be exported to distant sites through exosomal transport. The consequence of this interaction is far reaching especially in diseases such as cancer. For example, Luga and colleagues recently demonstrated that fibroblast-secreted exosomes promote breast cancer cell aggressiveness by protrusive activity and motility via Wnt-planar cell polarity signaling that was translated into enhanced metastasis [194]. These studies highlighted the role of intercellular communication pathway whereby fibroblast exosomes mobilize autocrine Wnt-planar cell polarity signaling to drive breast cancer invasive behavior.
8 Exosomes mediated drug expulsion, an emerging concept
Cytotoxic agents that target DNA integrity through intercalation or small molecule targeted agents that are designed against a protein cavity require proper cellular localization for their action [195]. Most drugs are administered intraveneously, or orally with the hope that in the biological systems they will reach to their target endpoint i.e. intracellular localization, resulting in efficient blocking of the pathway. However, cancer cells are smarter than originally believed because cancer cells exposed to drugs are recognized to expel drugs in extracellular compartments using specialized transporters of the multi-drug resistance (MDR)-ATP binding-cassette transporter (ABC transporters) system that are found to be activated in different malignancies [196]. MDR-ABC reversing agents have been implemented with some clinical success in patients, and thus the field of drug resistance has risen considerably over the last few decades [197]. However, most of the work related to drug export has been limited to ABC type transporters. Since exosome are recognized to carry ABC proteins, we propose that drugs or their intracellular metabolites can also be expelled out of the cells through exosomal mechanisms. The first indications on ABC transport mediated evasion from cancer therapy came from immunotherapy studies where it was shown that exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma is modulated by ATP-binding cassette transporter A3 (ABCA3) ([143] as discussed earlier). Below are some examples where exosome mediated drug resistance has been studied in different cancer models:
Using a prostate cancer model, Corcoran et al, showed that resistance to docetaxel was associated to enhance exosomal secretions, resulting in phenotypic changes in the morphology of the cells. In this study, MDR-1/P-gp exosomal transfer was considered as the primary driver for docetaxel resistance [198]. Further, exosomes from prostate cancer patients’ sera led to increased cell proliferation and invasion, compared to exosomes from age-matched controls. Although these studies fell short of evaluating the docetaxel content in the exosome, the findings do conclusively indicate that exosomes may play an important role in prostate cancer cell-cell communication, and thus may offer potential as vehicles containing predictive biomarkers and new therapeutic targets. In a very important study, Kerbey Shedden and colleagues investigated the encapsulation and expulsion of different anti-cancer drugs by exosomes/vesicles, and its correlation to drug sensitivity in different cancer models [199]. Using expression analysis for vesicle shedding genes in NCI 60 cell line panel, they demonstrated that both shedding index and GI50 (50% inhibitory drug concentrations) index for 171 compounds (drugs) were predominantly having positive relationships. These findings strongly indicated exosome/vesicle shedding and drug resistance are related across a broad spectrum of tumors. Most importantly, these authors was able to capture exosome-encapsulated doxorubicin using fluorescent microscopy, confirming the hypothesis that drugs are physically expelled by exosome type particles. Although few, yet these studies do support the idea that exosomal expulsion of drugs or their breakdown products may result in either reduced efficacy in cancer or even lead to its effect on non-target organs in adjacent tissues (Figure 4). However, more work is needed to delineate this novel mechanism of cellular exclusion of different drugs to solidify this hypothesis.
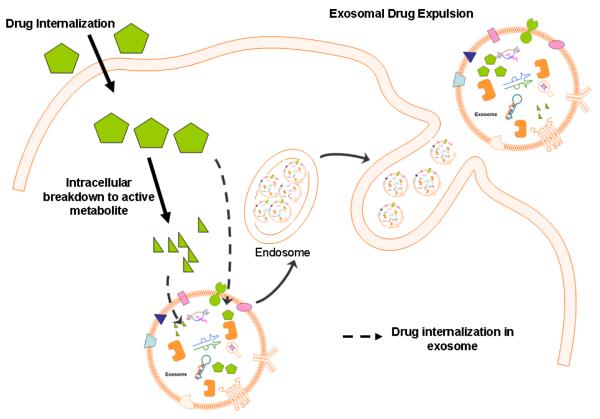
Figure-4. Drug Exclusion Mechanisms of Exosomes (hypothesis).
Diagram showing intracellular exosomal packaging of chemical drugs and or their breakdown products (active forms). Such exosome residing drugs can be expelled by cells, resulting in diminished drug efficacy and this is a distinctly separate from other drug transport mechanism.
9 Exosome Based Cancer Therapeutics
Exosome based therapies serve as attractive strategy against cancers and other diseases [200]. Being autologously generated within the host, they can be engineered to carry drugs or target proteins without invoking immunogenic response [201]. Exosome based delivery methods have been tested in the clinic successfully and were found to be well tolerated in patients (as discussed later). A number of different strategies have been applied to harness the potential of these exosomes, and are listed below. In the following section, we provide an overview of some of the different therapeutic applications of exosomes and also project the future course of therapeutics in cancer.
9.1 Exosome-based Immunotherapy for Cancer
The immune therapeutic benefits of exosomes were realized in the late 1990s. The first study highlighting the therapeutic role of exosomes in cancer came from the work of Zitvogel and colleagues where they showed that dendritic cells (DCs) secrete antigen presenting exosomes, having functional MHC class I and class II, and T-cell and co-stimulatory molecules [202]. These tumor peptide-pulsed DC-derived exosomes were found to prime specific cytotoxic T lymphocytes in vivo and eradicated or suppressed growth of established murine tumors in a T cell-dependent manner, suggesting that exosome-based cell-free vaccines could become an alternative to DC adoptive therapy against tumors [203]. Building on these studies, their team along with other colleagues investigated the molecular basis of exosome-induced immune stimulation, and analyzed the regulation of their production during DC maturation by characterizing the protein composition using peptide mass mapping [204]. In this investigation, exosomes were found to contain several cytosolic proteins (including annexin II, heat shock cognate protein hsc73, and heteromeric G protein Gi2alpha), as well as different integral or peripherally associated membrane proteins. Subcategorizing the components of secreted exosomes showed the presence of hsc73, a cytosolic heat shock protein (hsp) which is considered to be the primary factor inducing antitumor immune responses in vivo, and thus could be involved in the exosome’s potent antitumor effects. Interestingly their studies highlighted the suppressed production of exosomes during DC maturation, indicating that in the in vivo setting, exosomes are produced by immature DCs in peripheral tissues. Wolfer et al, using a human in vitro model system showed that exosomes, contain and transfer tumor antigens to dendritic cells [205]. In a mouse tumor exosome uptake model, the dendritic cells were shown to induce potent CD8+ T-cell-dependent antitumor effects on syngeneic and allogeneic established mouse tumors leading to their hypothesis that exosomes represent as a novel source of tumor-rejection antigens for T-cell cross priming, relevant for immuno-interventions. The above proof-of-concept studies paved the way for the production, isolation and characterization of clinical grade exosomes derived from dendritic cells [206]. Consequently, the tumor rejection potential of exosomes in CpG adjuvants efficiently primed with naive Tc1 lymphocytes was confirmed [207]. In another study, Taieb and colleagues demonstrated the synergistic effects of dendritic cell derived exosomes (DEX) on established mice tumors pretreated with immuno-potentiating doses of cyclophosphamide [208]. Here cyclophosphamide was shown to block the suppressive function of CD4+CD25+Foxp3+ regulatory T cells, and markedly enhanced the magnitude of secondary but not primary CTL responses induced by DEX vaccines. These findings built a strong case for therapeutic vaccines such as DEX aimed at boosting tumor-primed effector T cell. To improve the exosome-based tumor vaccines, Chen et al, investigated the efficacy of exosomes derived from heat-shocked mouse B-lymphoma cells (HS-Exo) in the induction of antitumor immune responses. Their studies indicated that HS-Exo, compared with control exosomes derived from the same cells (Exo), is enriched for HSP60 and HSP90. Importantly, HS-Exo were shown to induce dramatically increased antitumor immune responses compared to control Exo proving the increased potency of exosomes based vaccine over traditional vaccines.
9.2 Nanotechnology in Exosome therapeutic development
Being a nanoscale entity, exosomes have often been mostly viewed through the lens of nanotechnology [209]. Many different aspects of nanotechnology have been applied to either detect, characterize and even design exosome based therapies for different disease model systems [210]. For example, nano-tracking devices have been utilized to investigate and localize exosomes in different organisms [211;212]. Nano-based formulations that have been assembled to mimic exosomes have been projected as attractive therapeutics against cancer [213]. In this direction, Ristorcelli et al., developed pancreatic tumor nanoparticles that mimicked exosomes as characterized by proteomic analyses and rich in lipid rafts, decreased tumor cell proliferation [214]. This nano-exosome was shown to increase pro-apoptotic factors and suppressed anti-apoptotic Bcl-2, induced PTEN and glycogen synthase kinase (GSK)-3beta activation and decreased pyruvate dehydrogenase activity concurrently suppressing β-catenin. The same group showed that nano-formulation of exosome could induce pancreatic cancer cell death through the Notch pathway inhibition [57;100]. In another study, nano-injections of RNAi in dendritic derived exosomes allowed delivery to the brain without invoking immune response [215]. These findings were confirmed when Varez-Erviti et al., showed that siRNAs can be delivered across the blood brain barrier in a mouse model using systemic injections of exosomes [216]. Zhu and colleagues recently demonstrated that a magnetic iron oxide nanoparticles (MIONs)-induced exosomes can act as a signaling mediator in the induction of T helper cell type 1 (Th1) immune activation both in vitro and in in vivo in Balb/c mice model [217]. These are some of the studies highlighting the benefit of applying nano-based assays in the design of exosome drug therapies for cancer. As the characterization of exosome will evolve further, we expect to yield superior nano-formulations that could be designed against promoters of tumor microenvironment which may show treatment success against highly resistant cancer models.
9.3 Natural Agents (dietary and derivatives) and Exosomes
Plant derived dietary agents and their derivatives have been investigated for many decades for their health promoting benefits [218]. A number of agents have demonstrated anti-tumor activity in different animal models ranging from subcutaneous, orthotopic and advanced transgenic model system [219;220]. Being non-toxic, they serve as excellent agents that could be tested either for single agent efficacy or in adjuvant setting in the clinic. A number of diet derived agents have even been entered for clinical evaluation [221-223]. Generally speaking, most of natural agents such as resveratrol, curcumin, tea catechins (EGCG), etc. have pleiotropic mechanism of action [224]. These agents have well documented cancer cell selectivity and have also been shown to influence virtually most of the major signaling pathways, miRNA network and even hit epigenetic pathways. Likewise their efficacy on exosome release or against exosomes has also been tested in different laboratories independently. For example, Zhang and colleagues demonstrated that curcumin could partially suppress the exosomes-mediated inhibition of natural killer cells thereby reversing the sensitivity of breast tumor cells to different drugs [225]. In their study, pretreatment with curcumin impaired ubiquitin system, resulting in enhanced activation of STAT5 signaling. Additionally, Zhuang and colleagues utilized an exosome encapsulated curcumin formulation to demonstrate superior activity against brain inflammatory diseases [226]. These studies clearly point to an unexplored area of research where researchers can find answers to some of the unexplained mechanisms attributed to the multifaceted natural agents against cancer and other diseases.
9.4 Clinical status of Exosome based therapeutics and Future Challenges
Exosomes are well tolerated in humans, and have entered clinical trials for different cancers. Escudier et al., tested the feasibility and safety of autologous exosomes pulsed with MAGE 3 peptides for the immunization of stage III/IV melanoma patients in a Phase I clinical trial [227]. The secondary endpoints in this clinical study were (a) evaluation of T cell response and (b) clinical outcome. Interestingly, there was no grade II toxicity and the maximal tolerated dose was not achieved. One patient exhibited a partial response according to the RECIST criteria. In conclusion, the first exosome Phase I trial validated the feasibility of large scale exosome production for clinical administration in patients with melanoma. Recently a novel device based strategy was presented that involved extracorporeal hemofiltration of exosomes from the entire circulatory system using an affinity plasmapheresis platform known as the Aethlon ADAPT™ (adaptive dialysis-like affinity platform technology) system [228]. However, its clinical utility needs to be tested in future studies. At present, there are a number of clinical studies that utilize an exosomes based regimen (ClinicalTrials.gov website key word search Exosome). (1) NCT01550523 is a Phase I pilot immunotherapy trial for recurrent malignant gliomas that involves taking the patient’s own tumor cells during surgical craniotomy, treating them with an investigational new drug designed to shut down a targeted surface receptor protein, and re-implanting the cells. The study captures exosomes secreted from apoptotic cells that are rich in antigen titer. (2) NCT01159288 is a trial involving vaccination with tumor antigen-loaded dendritic cells derived exosomes. This trial uses second generation Dex against advanced unresectable NSCLC patients. (3) NCT01344109 is a pilot study of tumor-derived exosomes as diagnostic and prognostic markers in breast cancer patients receiving neoadjuvant chemotherapy. (4) NCT01668849 is a trial that involves natural agents (more specifically grape derived exosomes) to affect oral mucositis associated with chemo-radiation treatment of head and neck cancer. (5) NCT01779583 is a trial to investigate the circulating exosomes for their prognostic and predictive biomarker in gastric cancer patients, and (6) NCT01294072 is a study investigating the ability of plant exosomes to deliver effective doses of curcumin to normal and colon tumor tissue.
At present the miRNA based trials involving exosomes have not been initiated and we are still a long way from realizing their usefulness in the clinic because many critical challenges must be overcome to realize their therapeutic potential. For example, a number of studies have shown that all exosomes secreted by cells are not alike. In a colon model, Tauro and colleagues were able to capture two distinct types of exosomes [229] while in the human saliva, small RNA transcriptomes of different types of exosomes were recently captured using next generation sequencing [230]. The presence of distinct sub-population of exosomes adds to the complexity and further reiterates the need for a more robust search and characterization of these carriers in normal and disease models.
10 Conclusions and Future Direction
Success in treatment against complex cancers is dependent on our full understanding of the intricacies of interactions between different components within tumors. Exosomes are small particles with big functions and are emerging as major players in inter and intracellular communications. Their function in distant cell interaction is slowly being established and researchers have now been able to unwind their complex role in cancers. Exosomes have been shown to secrete diverse biological molecules ranging from virions, miRNAs, proteins and their complexes as presented here in the context of different therapeutics. There has been a drive to harness the potential of exosomes to deliver therapeutic content across biological membranes without invoking immune-response. The field of nanotechnology has extensively benefitted exosome research and researchers have been able to apply the nano principles to detect, characterize and load exosomes with warheads toward successful immunotherapy in clinical trials. Nevertheless, we are still a long way from fully realizing the power of these nano-scale carriers. Especially, little is known as to how they influence distant cell interactions within the tumor microenvironment (having multiple types of cells) that is a niche driving de novo and acquired drug resistance. This review discussed some of the aspects of exosome biology in solid tumor resistance where the multifaceted effects of these small carriers on promoting pathways to tumor resistance were explained. Most importantly, the role of miRNA in the context of exosome is an area of intense research and we sincerely hope that targeted inactivation of cancer causing miRNAs would likely provide newer strategy for targeting not only tumor cells but the entire tumor microenvironment to achieve cure for most cancers. It is hoped that researchers will dwell deeper into this emerging field of research and devise newer context dependent exosome based strategies to overcome therapeutic resistance, which happens to be the toughest and most challenging issue in cancer therapy.
Footnotes
Conflict of Interest Statements: None
Reference List
- 1.Kitano H. Cancer as a robust system: implications for anticancer therapy. Nat.Rev.Cancer. 2004;4:227–235. doi: 10.1038/nrc1300. [DOI] [PubMed] [Google Scholar]
- 2.Kitano H. Cancer robustness: tumour tactics. Nature. 2003;426:125. doi: 10.1038/426125a. [DOI] [PubMed] [Google Scholar]
- 3.Miyawaki A. Proteins on the move: insights gained from fluorescent protein technologies. Nat.Rev.Mol.Cell Biol. 2011;12:656–668. doi: 10.1038/nrm3199. [DOI] [PubMed] [Google Scholar]
- 4.Sugano K, Kansy M, Artursson P, Avdeef A, Bendels S, Di L, Ecker GF, Faller B, Fischer H, Gerebtzoff G, Lennernaes H, Senner F. Coexistence of passive and carrier-mediated processes in drug transport. Nat.Rev.Drug Discov. 2010;9:597–614. doi: 10.1038/nrd3187. [DOI] [PubMed] [Google Scholar]
- 5.El AS, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat.Rev.Drug Discov. 2013 doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 6.Staals RH, Pruijn GJ. The human exosome and disease. Adv.Exp.Med.Biol. 2010;702:132–142. [PubMed] [Google Scholar]
- 7.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim.Biophys.Acta. 2012;1820:940–948. doi: 10.1016/j.bbagen.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Vinciguerra P, Stutz F. mRNA export: an assembly line from genes to nuclear pores. Curr.Opin.Cell Biol. 2004;16:285–292. doi: 10.1016/j.ceb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Fevrier B, Vilette D, Laude H, Raposo G. Exosomes: a bubble ride for prions? Traffic. 2005;6:10–17. doi: 10.1111/j.1600-0854.2004.00247.x. [DOI] [PubMed] [Google Scholar]
- 11.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat.Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein A. CARRIER MODEL FOR ACTIVE TRANSPORT OF IONS ACROSS A MOSAIC MEMBRANE. Biophys.J. 1964;4:421–440. doi: 10.1016/s0006-3495(64)86793-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diekmann Y, Pereira-Leal JB. Evolution of intracellular compartmentalization. Biochem.J. 2013;449:319–331. doi: 10.1042/BJ20120957. [DOI] [PubMed] [Google Scholar]
- 14.Wright EM, Hirayama B, Hazama A, Loo DD, Supplisson S, Turk E, Hager KM. The sodium/glucose cotransporter (SGLT1) Soc.Gen.Physiol Ser. 1993;48:229–241. [PubMed] [Google Scholar]
- 15.Grunwald D, Singer RH, Rout M. Nuclear export dynamics of RNA-protein complexes. Nature. 2011;475:333–341. doi: 10.1038/nature10318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim J, Izadyar A, Nioradze N, Amemiya S. Nanoscale mechanism of molecular transport through the nuclear pore complex as studied by scanning electrochemical microscopy. J.Am.Chem.Soc. 2013;135:2321–2329. doi: 10.1021/ja311080j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albertini M, Pemberton LF, Rosenblum JS, Blobel G. A novel nuclear import pathway for the transcription factor TFIIS. J.Cell Biol. 1998;143:1447–1455. doi: 10.1083/jcb.143.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenblum JS, Pemberton LF, Bonifaci N, Blobel G. Nuclear import and the evolution of a multifunctional RNA-binding protein. J.Cell Biol. 1998;143:887–899. doi: 10.1083/jcb.143.4.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pemberton LF, Blobel G, Rosenblum JS. Transport routes through the nuclear pore complex. Curr.Opin.Cell Biol. 1998;10:392–399. doi: 10.1016/s0955-0674(98)80016-1. [DOI] [PubMed] [Google Scholar]
- 20.Rosenblum JS, Pemberton LF, Blobel G. A nuclear import pathway for a protein involved in tRNA maturation. J.Cell Biol. 1997;139:1655–1661. doi: 10.1083/jcb.139.7.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Jiko C, Yamashita E, Tsukihara T. Selective nuclear export mechanism of small RNAs. Curr.Opin.Struct.Biol. 2011;21:101–108. doi: 10.1016/j.sbi.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Adam SA, Lobl TJ, Mitchell MA, Gerace L. Identification of specific binding proteins for a nuclear location sequence. Nature. 1989;337:276–279. doi: 10.1038/337276a0. [DOI] [PubMed] [Google Scholar]
- 23.Wen W, Meinkoth JL, Tsien RY, Taylor SS. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 24.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J.Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 25.Trams EG, Lauter CJ, Salem N, Jr., Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochim.Biophys.Acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 26.van den Boorn JG, Dassler J, Coch C, Schlee M, Hartmann G. Exosomes as nucleic acid nanocarriers. Adv.Drug Deliv.Rev. 2013;65:331–335. doi: 10.1016/j.addr.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 27.Batista BS, Eng WS, Pilobello KT, Hendricks-Munoz KD, Mahal LK. Identification of a conserved glycan signature for microvesicles. J.Proteome.Res. 2011;10:4624–4633. doi: 10.1021/pr200434y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int.Immunol. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 29.Almqvist N, Lonnqvist A, Hultkrantz S, Rask C, Telemo E. Serum-derived exosomes from antigen-fed mice prevent allergic sensitization in a model of allergic asthma. Immunology. 2008;125:21–27. doi: 10.1111/j.1365-2567.2008.02812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CY, Hogan MC, Ward CJ. Purification of exosome-like vesicles from urine. Methods Enzymol. 2013;524:225–241. doi: 10.1016/B978-0-12-397945-2.00013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller S, Sanderson MP, Stoeck A, Altevogt P. Exosomes: from biogenesis and secretion to biological function. Immunol.Lett. 2006;107:102–108. doi: 10.1016/j.imlet.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J.Biol.Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y, El AS, Wood MJ. Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum.Mol.Genet. 2012;21:R125–R134. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 35.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr.Opin.Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Cocucci E, Racchetti G, Podini P, Meldolesi J. Enlargeosome traffic: exocytosis triggered by various signals is followed by endocytosis, membrane shedding or both. Traffic. 2007;8:742–757. doi: 10.1111/j.1600-0854.2007.00566.x. [DOI] [PubMed] [Google Scholar]
- 37.Cocucci E, Racchetti G, Rupnik M, Meldolesi J. The regulated exocytosis of enlargeosomes is mediated by a SNARE machinery that includes VAMP4. J.Cell Sci. 2008;121:2983–2991. doi: 10.1242/jcs.032029. [DOI] [PubMed] [Google Scholar]
- 38.Cocucci E, Meldolesi J. Ectosomes. Curr.Biol. 2011;21:R940–R941. doi: 10.1016/j.cub.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 39.Sudhof TC. The synaptic vesicle cycle. Annu.Rev.Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 40.Sudhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat.Cell Biol. 2010;12:19–30. doi: 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 42.Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, Ostrowski M, Thery C. Rab27a supports exosome-dependent and - independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–4930. doi: 10.1158/0008-5472.CAN-12-0925. [DOI] [PubMed] [Google Scholar]
- 43.Hsu C, Morohashi Y, Yoshimura S, Manrique-Hoyos N, Jung S, Lauterbach MA, Bakhti M, Gronborg M, Mobius W, Rhee J, Barr FA, Simons M. Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J.Cell Biol. 2010;189:223–232. doi: 10.1083/jcb.200911018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, Schwille P, Brugger B, Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 45.Parolini I, Federici C, Raggi C, Lugini L, Palleschi S, De MA, Coscia C, Iessi E, Logozzi M, Molinari A, Colone M, Tatti M, Sargiacomo M, Fais S. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J.Biol.Chem. 2009;284:34211–34222. doi: 10.1074/jbc.M109.041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen B, Fang Y, Wu N, Gould SJ. Biogenesis of the posterior pole is mediated by the exosome/microvesicle protein-sorting pathway. J.Biol.Chem. 2011;286:44162–44176. doi: 10.1074/jbc.M111.274803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poliakov A, Spilman M, Dokland T, Amling CL, Mobley JA. Structural heterogeneity and protein composition of exosome-like vesicles (prostasomes) in human semen. Prostate. 2009;69:159–167. doi: 10.1002/pros.20860. [DOI] [PubMed] [Google Scholar]
- 48.Simons M, Raposo G. Exosomes--vesicular carriers for intercellular communication. Curr.Opin.Cell Biol. 2009;21:575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 49.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J.Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 50.Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: proteomic insights and diagnostic potential. Expert.Rev.Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- 51.Gross JC, Boutros M. Secretion and extracellular space travel of Wnt proteins. Curr.Opin.Genet.Dev. 2013 doi: 10.1016/j.gde.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 52.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat.Cell Biol. 2012;14:1036–1045. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 53.Sheldon H, Heikamp E, Turley H, Dragovic R, Thomas P, Oon CE, Leek R, Edelmann M, Kessler B, Sainson RC, Sargent I, Li JL, Harris AL. New mechanism for Notch signaling to endothelium at a distance by Delta-like 4 incorporation into exosomes. Blood. 2010;116:2385–2394. doi: 10.1182/blood-2009-08-239228. [DOI] [PubMed] [Google Scholar]
- 54.Hasegawa H, Thomas HJ, Schooley K, Born TL. Native IL-32 is released from intestinal epithelial cells via a non-classical secretory pathway as a membrane-associated protein. Cytokine. 2011;53:74–83. doi: 10.1016/j.cyto.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Vidal M, Sainte-Marie J, Philippot JR, Bienvenue A. Asymmetric distribution of phospholipids in the membrane of vesicles released during in vitro maturation of guinea pig reticulocytes: evidence precluding a role for “aminophospholipid translocase”. J.Cell Physiol. 1989;140:455–462. doi: 10.1002/jcp.1041400308. [DOI] [PubMed] [Google Scholar]
- 56.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–212. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 57.Beloribi S, Ristorcelli E, Breuzard G, Silvy F, Bertrand-Michel J, Beraud E, Verine A, Lombardo D. Exosomal lipids impact notch signaling and induce death of human pancreatic tumoral SOJ-6 cells. PLoS.One. 2012;7:e47480. doi: 10.1371/journal.pone.0047480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, Kobayashi T, Salles JP, Perret B, Bonnerot C, Record M. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. Biochem.J. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuyama K, Sun H, Mitsutake S, Igarashi Y. Sphingolipid-modulated exosome secretion promotes clearance of amyloid-beta by microglia. J.Biol.Chem. 2012;287:10977–10989. doi: 10.1074/jbc.M111.324616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem.Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 61.Subra C, Grand D, Laulagnier K, Stella A, Lambeau G, Paillasse M, De MP, Monsarrat B, Perret B, Silvente-Poirot S, Poirot M, Record M. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J.Lipid Res. 2010;51:2105–2120. doi: 10.1194/jlr.M003657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Corrado C, Raimondo S, Chiesi A, Ciccia F, De LG, Alessandro R. Exosomes as intercellular signaling organelles involved in health and disease: basic science and clinical applications. Int.J.Mol.Sci. 2013;14:5338–5366. doi: 10.3390/ijms14035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mathivanan S, Fahner CJ, Reid GE, Simpson RJ. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 2012;40:D1241–D1244. doi: 10.1093/nar/gkr828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mathivanan S, Simpson RJ. ExoCarta: A compendium of exosomal proteins and RNA. Proteomics. 2009;9:4997–5000. doi: 10.1002/pmic.200900351. [DOI] [PubMed] [Google Scholar]
- 65.Geminard C, De GA, Vidal M. Reticulocyte maturation: mitoptosis and exosome release. Biocell. 2002;26:205–215. [PubMed] [Google Scholar]
- 66.Robertson C, Booth SA, Beniac DR, Coulthart MB, Booth TF, McNicol A. Cellular prion protein is released on exosomes from activated platelets. Blood. 2006;107:3907–3911. doi: 10.1182/blood-2005-02-0802. [DOI] [PubMed] [Google Scholar]
- 67.Quah B, O’Neill HC. Review: the application of dendritic cell-derived exosomes in tumour immunotherapy. Cancer Biother.Radiopharm. 2000;15:185–194. doi: 10.1089/cbr.2000.15.185. [DOI] [PubMed] [Google Scholar]
- 68.Denzer K, Kleijmeer MJ, Heijnen HF, Stoorvogel W, Geuze HJ. Exosome: from internal vesicle of the multivesicular body to intercellular signaling device. J.Cell Sci. 2000;113(Pt 19):3365–3374. doi: 10.1242/jcs.113.19.3365. [DOI] [PubMed] [Google Scholar]
- 69.Lasser C, Eldh M, Lotvall J. Isolation and characterization of RNA-containing exosomes. J.Vis.Exp. 2012:e3037. doi: 10.3791/3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gogolak P, Rethi B, Hajas G, Rajnavolgyi E. Targeting dendritic cells for priming cellular immune responses. J.Mol.Recognit. 2003;16:299–317. doi: 10.1002/jmr.650. [DOI] [PubMed] [Google Scholar]
- 71.Gyorgy B, Szabo TG, Pasztoi M, Pal Z, Misjak P, Aradi B, Laszlo V, Pallinger E, Pap E, Kittel A, Nagy G, Falus A, Buzas EI. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol.Life Sci. 2011;68:2667–2688. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vickers KC, Remaley AT. Lipid-based carriers of microRNAs and intercellular communication. Curr.Opin.Lipidol. 2012;23:91–97. doi: 10.1097/MOL.0b013e328350a425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaiswal R, Luk F, Gong J, Mathys JM, Grau GE, Bebawy M. Microparticle conferred microRNA profiles--implications in the transfer and dominance of cancer traits. Mol.Cancer. 2012;11:37. doi: 10.1186/1476-4598-11-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gong J, Jaiswal R, Mathys JM, Combes V, Grau GE, Bebawy M. Microparticles and their emerging role in cancer multidrug resistance. Cancer Treat.Rev. 2012;38:226–234. doi: 10.1016/j.ctrv.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 75.Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR. Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT) J.Proteome.Res. 2009;8:1304–1314. doi: 10.1021/pr800658c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Akerfelt M, Morimoto RI, Sistonen L. Heat shock factors: integrators of cell stress, development and lifespan. Nat.Rev.Mol.Cell Biol. 2010;11:545–555. doi: 10.1038/nrm2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akerfelt M, Trouillet D, Mezger V, Sistonen L. Heat shock factors at a crossroad between stress and development. Ann.N.Y.Acad.Sci. 2007;1113:15–27. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- 78.De MA. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11:1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- 79.Bolhassani A, Rafati S. Mini-chaperones: potential immuno-stimulators in vaccine design. Hum.Vaccin.Immunother. 2013;9:153–161. doi: 10.4161/hv.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert.Rev.Vaccines. 2008;7:1185–1199. doi: 10.1586/14760584.7.8.1185. [DOI] [PubMed] [Google Scholar]
- 81.Mathew A, Bell A, Johnstone RM. Hsp-70 is closely associated with the transferrin receptor in exosomes from maturing reticulocytes. Biochem.J. 1995;308(Pt 3):823–830. doi: 10.1042/bj3080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lancaster GI, Febbraio MA. Exosome-dependent trafficking of HSP70: a novel secretory pathway for cellular stress proteins. J.Biol.Chem. 2005;280:23349–23355. doi: 10.1074/jbc.M502017200. [DOI] [PubMed] [Google Scholar]
- 83.Clayton A, Turkes A, Navabi H, Mason MD, Tabi Z. Induction of heat shock proteins in B-cell exosomes. J.Cell Sci. 2005;118:3631–3638. doi: 10.1242/jcs.02494. [DOI] [PubMed] [Google Scholar]
- 84.Lv LH, Wan YL, Lin Y, Zhang W, Yang M, Li GL, Lin HM, Shang CZ, Chen YJ, Min J. Anticancer drugs cause release of exosomes with heat shock proteins from human hepatocellular carcinoma cells that elicit effective natural killer cell antitumor responses in vitro. J.Biol.Chem. 2012;287:15874–15885. doi: 10.1074/jbc.M112.340588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cho JA, Lee YS, Kim SH, Ko JK, Kim CW. MHC independent anti-tumor immune responses induced by Hsp70-enriched exosomes generate tumor regression in murine models. Cancer Lett. 2009;275:256–265. doi: 10.1016/j.canlet.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 86.Mukhopadhyay UK, Mak AS. p53: is the guardian of the genome also a suppressor of cell invasion? Cell Cycle. 2009;8:2481. doi: 10.4161/cc.8.16.9269. [DOI] [PubMed] [Google Scholar]
- 87.Muller PA, Vousden KH. p53 mutations in cancer. Nat.Cell Biol. 2013;15:2–8. doi: 10.1038/ncb2641. [DOI] [PubMed] [Google Scholar]
- 88.Wade M, Li YC, Wahl GM. MDM2, MDMX and p53 in oncogenesis and cancer therapy. Nat.Rev.Cancer. 2013;13:83–96. doi: 10.1038/nrc3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Azmi AS. Pharmaceutical reactivation of p53 pathways in cancer. Curr.Pharm.Des. 2011;17:534–535. doi: 10.2174/138161211795222630. [DOI] [PubMed] [Google Scholar]
- 90.Yu X, Harris SL, Levine AJ. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 2006;66:4795–4801. doi: 10.1158/0008-5472.CAN-05-4579. [DOI] [PubMed] [Google Scholar]
- 91.Yu X, Riley T, Levine AJ. The regulation of the endosomal compartment by p53 the tumor suppressor gene. FEBS J. 2009;276:2201–2212. doi: 10.1111/j.1742-4658.2009.06949.x. [DOI] [PubMed] [Google Scholar]
- 92.Lespagnol A, Duflaut D, Beekman C, Blanc L, Fiucci G, Marine JC, Vidal M, Amson R, Telerman A. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death.Differ. 2008;15:1723–1733. doi: 10.1038/cdd.2008.104. [DOI] [PubMed] [Google Scholar]
- 93.Honegger A, Leitz J, Bulkescher J, Hoppe-Seyler K, Hoppe-Seyler F. Silencing of human papillomavirus (HPV) E6/E7 oncogene expression affects both the contents and amounts of extracellular microvesicles released from HPV-positive cancer cells. Int.J.Cancer. 2013 doi: 10.1002/ijc.28164. [DOI] [PubMed] [Google Scholar]
- 94.Hupalowska A, Miaczynska M. The new faces of endocytosis in signaling. Traffic. 2012;13:9–18. doi: 10.1111/j.1600-0854.2011.01249.x. [DOI] [PubMed] [Google Scholar]
- 95.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat.Rev.Mol.Cell Biol. 2012;13:283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 96.Wrighton KH. Tumour suppressors: Role of nuclear PTEN revealed. Nat.Rev.Cancer. 2011;11:154. doi: 10.1038/nrc3028. [DOI] [PubMed] [Google Scholar]
- 97.Vanhaesebroeck B, Stephens L, Hawkins P. PI3K signalling: the path to discovery and understanding. Nat.Rev.Mol.Cell Biol. 2012;13:195–203. doi: 10.1038/nrm3290. [DOI] [PubMed] [Google Scholar]
- 98.Putz U, Howitt J, Doan A, Goh CP, Low LH, Silke J, Tan SS. The tumor suppressor PTEN is exported in exosomes and has phosphatase activity in recipient cells. Sci.Signal. 2012;5:ra70. doi: 10.1126/scisignal.2003084. [DOI] [PubMed] [Google Scholar]
- 99.Leslie NR. PTEN: an intercellular peacekeeper? Sci.Signal. 2012;5:e50. doi: 10.1126/scisignal.2003685. [DOI] [PubMed] [Google Scholar]
- 100.Ristorcelli E, Beraud E, Mathieu S, Lombardo D, Verine A. Essential role of Notch signaling in apoptosis of human pancreatic tumoral cells mediated by exosomal nanoparticles. Int.J.Cancer. 2009;125:1016–1026. doi: 10.1002/ijc.24375. [DOI] [PubMed] [Google Scholar]
- 101.Cho KR, Vogelstein B. Suppressor gene alterations in the colorectal adenoma-carcinoma sequence. J.Cell Biochem.Suppl. 1992;16G:137–141. doi: 10.1002/jcb.240501124. [DOI] [PubMed] [Google Scholar]
- 102.Aust DE, Terdiman JP, Willenbucher RF, Chang CG, Molinaro-Clark A, Baretton GB, Loehrs U, Waldman FM. The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas: a mutational analysis. Cancer. 2002;94:1421–1427. doi: 10.1002/cncr.10334. [DOI] [PubMed] [Google Scholar]
- 103.Lim JW, Mathias RA, Kapp EA, Layton MJ, Faux MC, Burgess AW, Ji H, Simpson RJ. Restoration of full-length APC protein in SW480 colon cancer cells induces exosome-mediated secretion of DKK-4. Electrophoresis. 2012;33:1873–1880. doi: 10.1002/elps.201100687. [DOI] [PubMed] [Google Scholar]
- 104.Seton-Rogers S. Microenvironment: Making connections. Nat.Rev.Cancer. 2013;13:222–223. doi: 10.1038/nrc3492. [DOI] [PubMed] [Google Scholar]
- 105.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat.Rev.Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 106.Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc.Natl.Acad.Sci.U.S.A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids--the mix of hormones and biomarkers. Nat.Rev.Clin.Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- 109.Stoorvogel W. Functional transfer of microRNA by exosomes. Blood. 2012;119:646–648. doi: 10.1182/blood-2011-11-389478. [DOI] [PubMed] [Google Scholar]
- 110.Gallo A, Tandon M, Alevizos I, Illei GG. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS.One. 2012;7:e30679. doi: 10.1371/journal.pone.0030679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Russo F, Di BS, Nigita G, Macca V, Lagana A, Giugno R, Pulvirenti A, Ferro A. miRandola: extracellular circulating microRNAs database. PLoS.One. 2012;7:e47786. doi: 10.1371/journal.pone.0047786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler.Thromb.Vasc.Biol. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat.Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 114.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh.Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Koga Y, Yasunaga M, Moriya Y, Akasu T, Fujita S, Yamamoto S, Matsumura Y. Exosome can prevent RNase from degrading microRNA in feces. J.Gastrointest.Oncol. 2011;2:215–222. doi: 10.3978/j.issn.2078-6891.2011.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen TS, Lai RC, Lee MM, Choo AB, Lee CN, Lim SK. Mesenchymal stem cell secretes microparticles enriched in pre-microRNAs. Nucleic Acids Res. 2010;38:215–224. doi: 10.1093/nar/gkp857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Flynt AS, Greimann JC, Chung WJ, Lima CD, Lai EC. MicroRNA biogenesis via splicing and exosome-mediated trimming in Drosophila. Mol.Cell. 2010;38:900–907. doi: 10.1016/j.molcel.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rabinowits G, Gercel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal microRNA: a diagnostic marker for lung cancer. Clin.Lung Cancer. 2009;10:42–46. doi: 10.3816/CLC.2009.n.006. [DOI] [PubMed] [Google Scholar]
- 119.Mizoguchi M, Guan Y, Yoshimoto K, Hata N, Amano T, Nakamizo A, Sasaki T. Clinical implications of microRNAs in human glioblastoma. Front Oncol. 2013;3:19. doi: 10.3389/fonc.2013.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tanaka Y, Kamohara H, Kinoshita K, Kurashige J, Ishimoto T, Iwatsuki M, Watanabe M, Baba H. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119:1159–1167. doi: 10.1002/cncr.27895. [DOI] [PubMed] [Google Scholar]
- 121.Hessvik NP, Sandvig K, Llorente A. Exosomal miRNAs as Biomarkers for Prostate Cancer. Front Genet. 2013;4:36. doi: 10.3389/fgene.2013.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hessvik NP, Phuyal S, Brech A, Sandvig K, Llorente A. Profiling of microRNAs in exosomes released from PC-3 prostate cancer cells. Biochim.Biophys.Acta. 2012;1819:1154–1163. doi: 10.1016/j.bbagrm.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 123.da Silveira JC, Veeramachaneni DN, Winger QA, Carnevale EM, Bouma GJ. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biol.Reprod. 2012;86:71. doi: 10.1095/biolreprod.111.093252. [DOI] [PubMed] [Google Scholar]
- 124.Lasser C. Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert.Opin.Biol.Ther. 2012;12(Suppl 1):S189–S197. doi: 10.1517/14712598.2012.680018. [DOI] [PubMed] [Google Scholar]
- 125.Tauro BJ, Greening DW, Mathias RA, Ji H, Mathivanan S, Scott AM, Simpson RJ. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods. 2012;56:293–304. doi: 10.1016/j.ymeth.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 126.Alvarez ML, Khosroheidari M, Kanchi RR, DiStefano JK. Comparison of protein, microRNA, and mRNA yields using different methods of urinary exosome isolation for the discovery of kidney disease biomarkers. Kidney Int. 2012;82:1024–1032. doi: 10.1038/ki.2012.256. [DOI] [PubMed] [Google Scholar]
- 127.Umezu T, Ohyashiki K, Kuroda M, Ohyashiki JH. Leukemia cell to endothelial cell communication via exosomal miRNAs. Oncogene. 2012 doi: 10.1038/onc.2012.295. [DOI] [PubMed] [Google Scholar]
- 128.Bobrie A, Colombo M, Raposo G, Thery C. Exosome secretion: molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–1668. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 129.Pegtel DM, van d.G., Middeldorp JM. Viral miRNAs exploiting the endosomal-exosomal pathway for intercellular cross-talk and immune evasion. Biochim.Biophys.Acta. 2011;1809:715–721. doi: 10.1016/j.bbagrm.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Palma J, Yaddanapudi SC, Pigati L, Havens MA, Jeong S, Weiner GA, Weimer KM, Stern B, Hastings ML, Duelli DM. MicroRNAs are exported from malignant cells in customized particles. Nucleic Acids Res. 2012;40:9125–9138. doi: 10.1093/nar/gks656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang M, Chen J, Su F, Yu B, Su F, Lin L, Liu Y, Huang JD, Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells. Mol.Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bullerdiek J, Flor I. Exosome-delivered microRNAs of “chromosome 19 microRNA cluster” as immunomodulators in pregnancy and tumorigenesis. Mol.Cytogenet. 2012;5:27. doi: 10.1186/1755-8166-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Meads MB, Gatenby RA, Dalton WS. Environment-mediated drug resistance: a major contributor to minimal residual disease. Nat.Rev.Cancer. 2009;9:665–674. doi: 10.1038/nrc2714. [DOI] [PubMed] [Google Scholar]
- 134.Shain KH, Landowski TH, Dalton WS. The tumor microenvironment as a determinant of cancer cell survival: a possible mechanism for de novo drug resistance. Curr.Opin.Oncol. 2000;12:557–563. doi: 10.1097/00001622-200011000-00008. [DOI] [PubMed] [Google Scholar]
- 135.Li H, Yang BB. Friend or foe: the role of microRNA in chemotherapy resistance. Acta Pharmacol.Sin. 2013 doi: 10.1038/aps.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holzel M, Bovier A, Tuting T. Plasticity of tumour and immune cells: a source of heterogeneity and a cause for therapy resistance? Nat.Rev.Cancer. 2013;13:365–376. doi: 10.1038/nrc3498. [DOI] [PubMed] [Google Scholar]
- 137.McMillin DW, Negri JM, Mitsiades CS. The role of tumour-stromal interactions in modifying drug response: challenges and opportunities. Nat.Rev.Drug Discov. 2013;12:217–228. doi: 10.1038/nrd3870. [DOI] [PubMed] [Google Scholar]
- 138.Khan S, Aspe JR, Asumen MG, Almaguel F, Odumosu O, cevedo-Martinez S, De LM, Langridge WH, Wall NR. Extracellular, cell-permeable survivin inhibits apoptosis while promoting proliferative and metastatic potential. Br.J.Cancer. 2009;100:1073–1086. doi: 10.1038/sj.bjc.6604978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pilzer D, Gasser O, Moskovich O, Schifferli JA, Fishelson Z. Emission of membrane vesicles: roles in complement resistance, immunity and cancer. Springer Semin.Immunopathol. 2005;27:375–387. doi: 10.1007/s00281-005-0004-1. [DOI] [PubMed] [Google Scholar]
- 140.Pilzer D, Fishelson Z. Mortalin/GRP75 promotes release of membrane vesicles from immune attacked cells and protection from complement-mediated lysis. Int.Immunol. 2005;17:1239–1248. doi: 10.1093/intimm/dxh300. [DOI] [PubMed] [Google Scholar]
- 141.Zhang HG, Liu C, Su K, Yu S, Zhang L, Zhang S, Wang J, Cao X, Grizzle W, Kimberly RP. A membrane form of TNF-alpha presented by exosomes delays T cell activation-induced cell death. J.Immunol. 2006;176:7385–7393. doi: 10.4049/jimmunol.176.12.7385. [DOI] [PubMed] [Google Scholar]
- 142.Bodey B, Bodey B, Jr., Kaiser HE. Dendritic type, accessory cells within the mammalian thymic microenvironment. Antigen presentation in the dendritic neuro-endocrine-immune cellular network. In Vivo. 1997;11:351–370. [PubMed] [Google Scholar]
- 143.Aung T, Chapuy B, Vogel D, Wenzel D, Oppermann M, Lahmann M, Weinhage T, Menck K, Hupfeld T, Koch R, Trumper L, Wulf GG. Exosomal evasion of humoral immunotherapy in aggressive B-cell lymphoma modulated by ATP-binding cassette transporter A3. Proc.Natl.Acad.Sci.U.S.A. 2011;108:15336–15341. doi: 10.1073/pnas.1102855108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hupfeld T, Chapuy B, Schrader V, Beutler M, Veltkamp C, Koch R, Cameron S, Aung T, Haase D, Larosee P, Truemper L, Wulf GG. Tyrosinekinase inhibition facilitates cooperation of transcription factor SALL4 and ABC transporter A3 towards intrinsic CML cell drug resistance. Br.J.Haematol. 2013;161:204–213. doi: 10.1111/bjh.12246. [DOI] [PubMed] [Google Scholar]
- 145.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, Howell SB. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Mol.Cancer Ther. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 146.Yin J, Yan X, Yao X, Zhang Y, Shan Y, Mao N, Yang Y, Pan L. Secretion of annexin A3 from ovarian cancer cells and its association with platinum resistance in ovarian cancer patients. J.Cell Mol.Med. 2012;16:337–348. doi: 10.1111/j.1582-4934.2011.01316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ciravolo V, Huber V, Ghedini GC, Venturelli E, Bianchi F, Campiglio M, Morelli D, Villa A, Della MP, Menard S, Filipazzi P, Rivoltini L, Tagliabue E, Pupa SM. Potential role of HER2-overexpressing exosomes in countering trastuzumab-based therapy. J.Cell Physiol. 2012;227:658–667. doi: 10.1002/jcp.22773. [DOI] [PubMed] [Google Scholar]
- 148.Hosseini-Beheshti E, Pham S, Adomat H, Li N, Tomlinson Guns ES. Exosomes as biomarker enriched microvesicles: characterization of exosomal proteins derived from a panel of prostate cell lines with distinct AR phenotypes. Mol.Cell Proteomics. 2012;11:863–885. doi: 10.1074/mcp.M111.014845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bard MP, Hegmans JP, Hemmes A, Luider TM, Willemsen R, Severijnen LA, van Meerbeeck JP, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am.J.Respir.Cell Mol.Biol. 2004;31:114–121. doi: 10.1165/rcmb.2003-0238OC. [DOI] [PubMed] [Google Scholar]
- 150.Hegmans JP, Bard MP, Hemmes A, Luider TM, Kleijmeer MJ, Prins JB, Zitvogel L, Burgers SA, Hoogsteden HC, Lambrecht BN. Proteomic analysis of exosomes secreted by human mesothelioma cells. Am.J.Pathol. 2004;164:1807–1815. doi: 10.1016/S0002-9440(10)63739-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, Patel P, Selby PJ, Banks RE. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 152.Nguyen DX, Bos PD, Massague J. Metastasis: from dissemination to organ-specific colonization. Nat.Rev.Cancer. 2009;9:274–284. doi: 10.1038/nrc2622. [DOI] [PubMed] [Google Scholar]
- 153.Nguyen DX, Massague J. Genetic determinants of cancer metastasis. Nat.Rev.Genet. 2007;8:341–352. doi: 10.1038/nrg2101. [DOI] [PubMed] [Google Scholar]
- 154.Grange C, Tapparo M, Collino F, Vitillo L, Damasco C, Deregibus MC, Tetta C, Bussolati B, Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.CAN-11-0241. [DOI] [PubMed] [Google Scholar]
- 155.Hood JL, Pan H, Lanza GM, Wickline SA. Paracrine induction of endothelium by tumor exosomes. Lab Invest. 2009;89:1317–1328. doi: 10.1038/labinvest.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hood JL, San RS, Wickline SA. Exosomes released by melanoma cells prepare sentinel lymph nodes for tumor metastasis. Cancer Res. 2011;71:3792–3801. doi: 10.1158/0008-5472.CAN-10-4455. [DOI] [PubMed] [Google Scholar]
- 157.Rana S, Malinowska K, Zoller M. Exosomal tumor microRNA modulates premetastatic organ cells. Neoplasia. 2013;15:281–295. doi: 10.1593/neo.122010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Di VD, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, Souza-Schorey C, Freeman MR. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am.J.Pathol. 2012;181:1573–1584. doi: 10.1016/j.ajpath.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bao B, Azmi AS, Ali S, Ahmad A, Li Y, Banerjee S, Kong D, Sarkar FH. The biological kinship of hypoxia with CSC and EMT and their relationship with deregulated expression of miRNAs and tumor aggressiveness. Biochim.Biophys.Acta. 2012;1826:272–296. doi: 10.1016/j.bbcan.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Casazza A, Di CG, Wenes M, Finisguerra V, Deschoemaeker S, Mazzone M. Tumor stroma: a complexity dictated by the hypoxic tumor microenvironment. Oncogene. 2013 doi: 10.1038/onc.2013.121. [DOI] [PubMed] [Google Scholar]
- 161.Salnikov AV, Liu L, Platen M, Gladkich J, Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J, Schemmer P, Buchler MW, Herr I. Hypoxia induces EMT in low and highly aggressive pancreatic tumor cells but only cells with cancer stem cell characteristics acquire pronounced migratory potential. PLoS.One. 2012;7:e46391. doi: 10.1371/journal.pone.0046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chaturvedi P, Gilkes DM, Wong CC, Luo W, Zhang H, Wei H, Takano N, Schito L, Levchenko A, Semenza GL. Hypoxia-inducible factor-dependent breast cancer-mesenchymal stem cell bidirectional signaling promotes metastasis. J.Clin.Invest. 2013;123:189–205. doi: 10.1172/JCI64993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat.Rev.Cancer. 2011;11:393–410. doi: 10.1038/nrc3064. [DOI] [PubMed] [Google Scholar]
- 164.Rapisarda A, Melillo G. Overcoming disappointing results with antiangiogenic therapy by targeting hypoxia. Nat.Rev.Clin.Oncol. 2012;9:378–390. doi: 10.1038/nrclinonc.2012.64. [DOI] [PubMed] [Google Scholar]
- 165.King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC.Cancer. 2012;12:421. doi: 10.1186/1471-2407-12-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kucharzewska P, Christianson HC, Welch JE, Svensson KJ, Fredlund E, Ringner M, Morgelin M, Bourseau-Guilmain E, Bengzon J, Belting M. Exosomes reflect the hypoxic status of glioma cells and mediate hypoxia-dependent activation of vascular cells during tumor development. Proc.Natl.Acad.Sci.U.S.A. 2013 doi: 10.1073/pnas.1220998110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Borges FT, Melo SA, Ozdemir BC, Kato N, Revuelta I, Miller CA, Gattone VH, LeBleu VS, Kalluri R. TGF-beta1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J.Am.Soc.Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol.Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Svensson KJ, Kucharzewska P, Christianson HC, Skold S, Lofstedt T, Johansson MC, Morgelin M, Bengzon J, Ruf W, Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc.Natl.Acad.Sci.U.S.A. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Svensson KJ, Belting M. Role of extracellular membrane vesicles in intercellular communication of the tumour microenvironment. Biochem.Soc.Trans. 2013;41:273–276. doi: 10.1042/BST20120248. [DOI] [PubMed] [Google Scholar]
- 171.Park JE, Tan HS, Datta A, Lai RC, Zhang H, Meng W, Lim SK, Sze SK. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol.Cell Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat.Rev.Mol.Cell Biol. 2006;7:131–142. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 173.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat.Rev.Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 174.Masuda S, Izpisua Belmonte JC. The microenvironment and resistance to personalized cancer therapy. Nat.Rev.Clin.Oncol. 2013;10 doi: 10.1038/nrclinonc.2012.127-c1. [DOI] [PubMed] [Google Scholar]
- 175.Garnier D, Magnus N, Lee TH, Bentley V, Meehan B, Milsom C, Montermini L, Kislinger T, Rak J. Cancer cells induced to express mesenchymal phenotype release exosome-like extracellular vesicles carrying tissue factor. J.Biol.Chem. 2012;287:43565–43572. doi: 10.1074/jbc.M112.401760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Roccaro AM, Sacco A, Maiso P, Azab AK, Tai YT, Reagan M, Azab F, Flores LM, Campigotto F, Weller E, Anderson KC, Scadden DT, Ghobrial IM. BM mesenchymal stromal cell-derived exosomes facilitate multiple myeloma progression. J.Clin.Invest. 2013 doi: 10.1172/JCI66517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Katsuno Y, Lamouille S, Derynck R. TGF-beta signaling and epithelial-mesenchymal transition in cancer progression. Curr.Opin.Oncol. 2013;25:76–84. doi: 10.1097/CCO.0b013e32835b6371. [DOI] [PubMed] [Google Scholar]
- 178.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19:156–172. doi: 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lamouille S, Connolly E, Smyth JW, Akhurst RJ, Derynck R. TGF-beta-induced activation of mTOR complex 2 drives epithelial-mesenchymal transition and cell invasion. J.Cell Sci. 2012;125:1259–1273. doi: 10.1242/jcs.095299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cho JA, Park H, Lim EH, Lee KW. Exosomes from breast cancer cells can convert adipose tissue-derived mesenchymal stem cells into myofibroblast-like cells. Int.J.Oncol. 2012;40:130–138. doi: 10.3892/ijo.2011.1193. [DOI] [PubMed] [Google Scholar]
- 181.Clayton A, Mitchell JP, Court J, Mason MD, Tabi Z. Human tumor-derived exosomes selectively impair lymphocyte responses to interleukin-2. Cancer Res. 2007;67:7458–7466. doi: 10.1158/0008-5472.CAN-06-3456. [DOI] [PubMed] [Google Scholar]
- 182.Cheng CF, Fan J, Fedesco M, Guan S, Li Y, Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, Han YP, Woodley DT, Li W. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol.Cell Biol. 2008;28:3344–3358. doi: 10.1128/MCB.01287-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Clayton A, Mitchell JP, Court J, Linnane S, Mason MD, Tabi Z. Human tumor-derived exosomes down-modulate NKG2D expression. J.Immunol. 2008;180:7249–7258. doi: 10.4049/jimmunol.180.11.7249. [DOI] [PubMed] [Google Scholar]
- 184.Cho JA, Park H, Lim EH, Kim KH, Choi JS, Lee JH, Shin JW, Lee KW. Exosomes from ovarian cancer cells induce adipose tissue-derived mesenchymal stem cells to acquire the physical and functional characteristics of tumor-supporting myofibroblasts. Gynecol.Oncol. 2011;123:379–386. doi: 10.1016/j.ygyno.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 185.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat.Rev.Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 186.Anastas JN, Moon RT. WNT signalling pathways as therapeutic targets in cancer. Nat.Rev.Cancer. 2013;13:11–26. doi: 10.1038/nrc3419. [DOI] [PubMed] [Google Scholar]
- 187.Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development. 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- 188.Morin PJ. beta-catenin signaling and cancer. Bioessays. 1999;21:1021–1030. doi: 10.1002/(SICI)1521-1878(199912)22:1<1021::AID-BIES6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 189.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Koles K, Nunnari J, Korkut C, Barria R, Brewer C, Li Y, Leszyk J, Zhang B, Budnik V. Mechanism of evenness interrupted (Evi)-exosome release at synaptic boutons. J.Biol.Chem. 2012;287:16820–16834. doi: 10.1074/jbc.M112.342667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Chairoungdua A, Smith DL, Pochard P, Hull M, Caplan MJ. Exosome release of beta-catenin: a novel mechanism that antagonizes Wnt signaling. J.Cell Biol. 2010;190:1079–1091. doi: 10.1083/jcb.201002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li D, Ren YN, Yang J, Yang YM, Li CY, Xie RF, Fan HH. A preliminary study on the influence of human plasma exosomes-like vesicles on macrophage Wnt5A-Ca(2)+ pathway. Zhonghua Xue.Ye.Xue.Za Zhi. 2011;32:404–407. [PubMed] [Google Scholar]
- 193.Hooper C, Sainz-Fuertes R, Lynham S, Hye A, Killick R, Warley A, Bolondi C, Pocock J, Lovestone S. Wnt3a induces exosome secretion from primary cultured rat microglia. BMC.Neurosci. 2012;13:144. doi: 10.1186/1471-2202-13-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL. Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration. Cell. 2012;151:1542–1556. doi: 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- 195.Stumpf WE. Drug localization and targeting with receptor microscopic autoradiography. J.Pharmacol.Toxicol.Methods. 2005;51:25–40. doi: 10.1016/j.vascn.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 196.Jones PM, George AM. The ABC transporter structure and mechanism: perspectives on recent research. Cell Mol.Life Sci. 2004;61:682–699. doi: 10.1007/s00018-003-3336-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lee CH. Reversing agents for ATP-binding cassette drug transporters. Methods Mol.Biol. 2010;596:325–340. doi: 10.1007/978-1-60761-416-6_14. [DOI] [PubMed] [Google Scholar]
- 198.Corcoran C, Rani S, O’Brien K, O’Neill A, Prencipe M, Sheikh R, Webb G, McDermott R, Watson W, Crown J, O’Driscoll L. Docetaxel-resistance in prostate cancer: evaluating associated phenotypic changes and potential for resistance transfer via exosomes. PLoS.One. 2012;7:e50999. doi: 10.1371/journal.pone.0050999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Shedden K, Xie XT, Chandaroy P, Chang YT, Rosania GR. Expulsion of small molecules in vesicles shed by cancer cells: association with gene expression and chemosensitivity profiles. Cancer Res. 2003;63:4331–4337. [PubMed] [Google Scholar]
- 200.Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM. Exosome mimetics: a novel class of drug delivery systems. Int.J.Nanomedicine. 2012;7:1525–1541. doi: 10.2147/IJN.S29661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Harding CV, Heuser JE, Stahl PD. Exosomes: looking back three decades and into the future. J.Cell Biol. 2013;200:367–371. doi: 10.1083/jcb.201212113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nat.Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 203.Zitvogel L, Fernandez N, Lozier A, Wolfers J, Regnault A, Raposo G, Amigorena S. Dendritic cells or their exosomes are effective biotherapies of cancer. Eur.J.Cancer. 1999;35(Suppl 3):S36–S38. doi: 10.1016/s0959-8049(99)00090-8. [DOI] [PubMed] [Google Scholar]
- 204.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. J.Cell Biol. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wolfers J, Lozier A, Raposo G, Regnault A, Thery C, Masurier C, Flament C, Pouzieux S, Faure F, Tursz T, Angevin E, Amigorena S, Zitvogel L. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat.Med. 2001;7:297–303. doi: 10.1038/85438. [DOI] [PubMed] [Google Scholar]
- 206.Lamparski HG, Metha-Damani A, Yao JY, Patel S, Hsu DH, Ruegg C, Le Pecq JB. Production and characterization of clinical grade exosomes derived from dendritic cells. J.Immunol.Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 207.Chaput N, Schartz NE, Andre F, Taieb J, Novault S, Bonnaventure P, Aubert N, Bernard J, Lemonnier F, Merad M, Adema G, Adams M, Ferrantini M, Carpentier AF, Escudier B, Tursz T, Angevin E, Zitvogel L. Exosomes as potent cell-free peptide-based vaccine. II. Exosomes in CpG adjuvants efficiently prime naive Tc1 lymphocytes leading to tumor rejection. J.Immunol. 2004;172:2137–2146. doi: 10.4049/jimmunol.172.4.2137. [DOI] [PubMed] [Google Scholar]
- 208.Taieb J, Chaput N, Schartz N, Roux S, Novault S, Menard C, Ghiringhelli F, Terme M, Carpentier AF, rrasse-Jeze G, Lemonnier F, Zitvogel L. Chemoimmunotherapy of tumors: cyclophosphamide synergizes with exosome based vaccines. J.Immunol. 2006;176:2722–2729. doi: 10.4049/jimmunol.176.5.2722. [DOI] [PubMed] [Google Scholar]
- 209.Tian X, Zhu M, Nie G. How can nanotechnology help membrane vesicle-based cancer immunotherapy development? Hum.Vaccin.Immunother. 2013;9:222–225. doi: 10.4161/hv.22130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hood JL, Wickline SA. A systematic approach to exosome-based translational nanomedicine. Wiley.Interdiscip.Rev.Nanomed.Nanobiotechnol. 2012;4:458–467. doi: 10.1002/wnan.1174. [DOI] [PubMed] [Google Scholar]
- 211.Sokolova V, Ludwig AK, Hornung S, Rotan O, Horn PA, Epple M, Giebel B. Characterisation of exosomes derived from human cells by nanoparticle tracking analysis and scanning electron microscopy. Colloids Surf.B Biointerfaces. 2011;87:146–150. doi: 10.1016/j.colsurfb.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 212.Marchesano V, Hernandez Y, Salvenmoser W, Ambrosone A, Tino A, Hobmayer B, la Fuente M.d., Tortiglione C. Imaging inward and outward trafficking of gold nanoparticles in whole animals. ACS Nano. 2013;7:2431–2442. doi: 10.1021/nn305747e. [DOI] [PubMed] [Google Scholar]
- 213.Tan A, De La PH, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. Int.J.Nanomedicine. 2010;5:889–900. doi: 10.2147/IJN.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ristorcelli E, Beraud E, Verrando P, Villard C, Lafitte D, Sbarra V, Lombardo D, Verine A. Human tumor nanoparticles induce apoptosis of pancreatic cancer cells. FASEB J. 2008;22:3358–3369. doi: 10.1096/fj.07-102855. [DOI] [PubMed] [Google Scholar]
- 215.Lakhal S, Wood MJ. Exosome nanotechnology: an emerging paradigm shift in drug delivery: exploitation of exosome nanovesicles for systemic in vivo delivery of RNAi heralds new horizons for drug delivery across biological barriers. Bioessays. 2011;33:737–741. doi: 10.1002/bies.201100076. [DOI] [PubMed] [Google Scholar]
- 216.varez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat.Biotechnol. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]
- 217.Zhu M, Tian X, Song X, Li Y, Tian Y, Zhao Y, Nie G. Nanoparticle-induced exosomes target antigen-presenting cells to initiate Th1-type immune activation. Small. 2012;8:2841–2848. doi: 10.1002/smll.201200381. [DOI] [PubMed] [Google Scholar]
- 218.Halliwell B. Dietary polyphenols: good, bad, or indifferent for your health? Cardiovasc.Res. 2007;73:341–347. doi: 10.1016/j.cardiores.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 219.Raffoul JJ, Kucuk O, Sarkar FH, Hillman GG. Dietary agents in cancer chemoprevention and treatment. J.Oncol. 2012;2012:749310. doi: 10.1155/2012/749310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat.Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Asher GN, Spelman K. Clinical utility of curcumin extract. Altern.Ther.Health Med. 2013;19:20–22. [PubMed] [Google Scholar]
- 222.Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, Cher ML, Andic F, Rossi PJ, Kucuk O. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr.Cancer. 2010;62:996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Heath EI, Heilbrun LK, Li J, Vaishampayan U, Harper F, Pemberton P, Sarkar FH. A phase I dose-escalation study of oral BR-DIM (BioResponse 3,3′-Diindolylmethane) in castrate-resistant, non-metastatic prostate cancer. Am.J.Transl.Res. 2010;2:402–411. [PMC free article] [PubMed] [Google Scholar]
- 224.Ahmad A, Sakr WA, Rahman KM. Novel targets for detection of cancer and their modulation by chemopreventive natural compounds. Front Biosci.(Elite.Ed) 2012;4:410–425. doi: 10.2741/e388. [DOI] [PubMed] [Google Scholar]
- 225.Zhang HG, Kim H, Liu C, Yu S, Wang J, Grizzle WE, Kimberly RP, Barnes S. Curcumin reverses breast tumor exosomes mediated immune suppression of NK cell tumor cytotoxicity. Biochim.Biophys.Acta. 2007;1773:1116–1123. doi: 10.1016/j.bbamcr.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zhuang X, Xiang X, Grizzle W, Sun D, Zhang S, Axtell RC, Ju S, Mu J, Zhang L, Steinman L, Miller D, Zhang HG. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol.Ther. 2011;19:1769–1779. doi: 10.1038/mt.2011.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J.Transl.Med. 2005;3:10. doi: 10.1186/1479-5876-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Marleau AM, Chen CS, Joyce JA, Tullis RH. Exosome removal as a therapeutic adjuvant in cancer. J.Transl.Med. 2012;10:134. doi: 10.1186/1479-5876-10-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Tauro BJ, Greening DW, Mathias RA, Mathivanan S, Ji H, Simpson RJ. Two distinct populations of exosomes are released from LIM1863 colon carcinoma cell-derived organoids. Mol.Cell Proteomics. 2013;12:587–598. doi: 10.1074/mcp.M112.021303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ogawa Y, Taketomi Y, Murakami M, Tsujimoto M, Yanoshita R. Small RNA transcriptomes of two types of exosomes in human whole saliva determined by next generation sequencing. Biol.Pharm.Bull. 2013;36:66–75. doi: 10.1248/bpb.b12-00607. [DOI] [PubMed] [Google Scholar]