Figure 1.
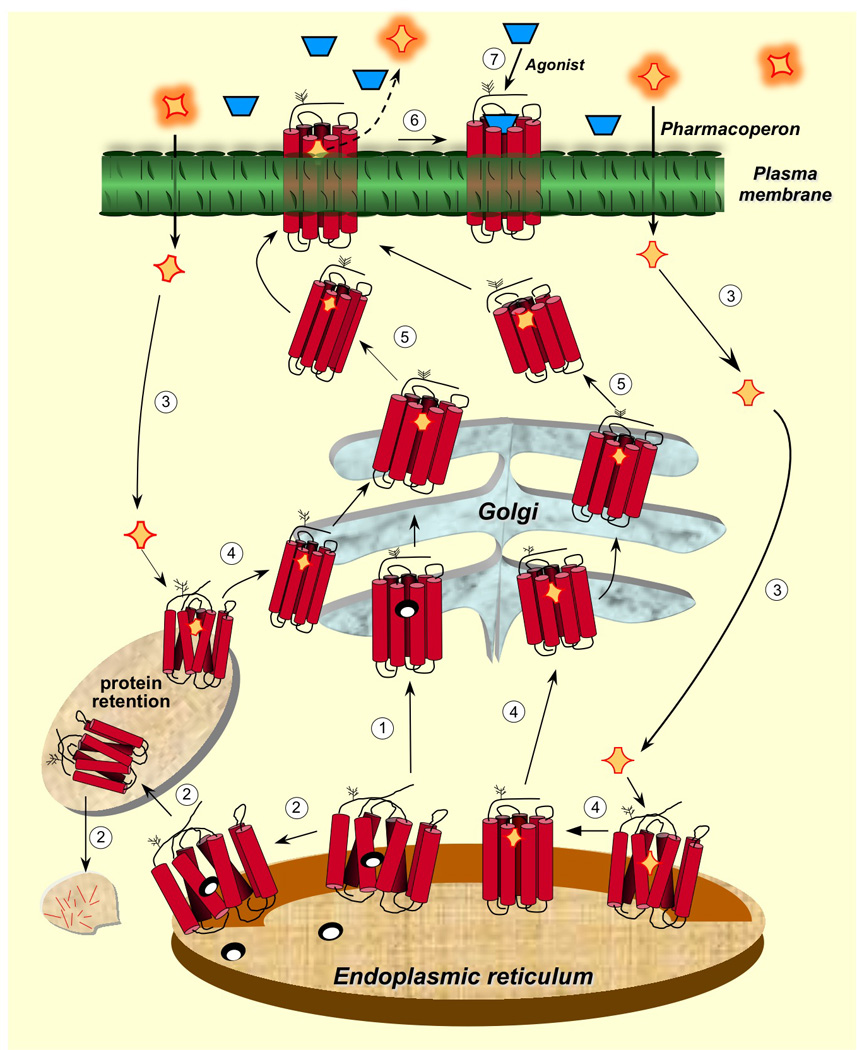
Intracellular trafficking of GPCRs from the ER to the plasma membrane and relation to the cellular quality control system. Newly synthesized proteins are translocated to the lumen of the ER where folding is both facilitated and/or corrected by molecular chaperones (oval structures). The correctly folded protein is thereafter exported to the Golgi complex for further processing (eg. glycosylation) (step 1 in clear circles) and routing to the plasma membrane. When folding fails, misfolded proteins are retained in the ER and eventually targeted for degradation through the polyubiquitination/proteasome pathway (step 2). Pharmacoperones (orange rhomboid structures) diffuse into the cell (step 3) and selectively bind the misfolded protein to correct misfolding promoting its export to the Golgi complex (step 4). Previously synthesized misfolded proteins, retained by the QCS, may still be rescued by pharmacoperones (steps 3 and 4 at the left). Mature processed proteins are then delivered to the cell surface PM (step 5), where the pharmacoperone can dissociate from the target protein (step 6) allowing the receptor to interact with agonist (step 7). Development of pharmacoperones that dissociate functions or rescue from agonism is currently needed as many pharmacoperone drugs are also receptor agonists/antagonists. Modified from Conn and Ulloa-Aguirre (2011), with permission of the publisher.
