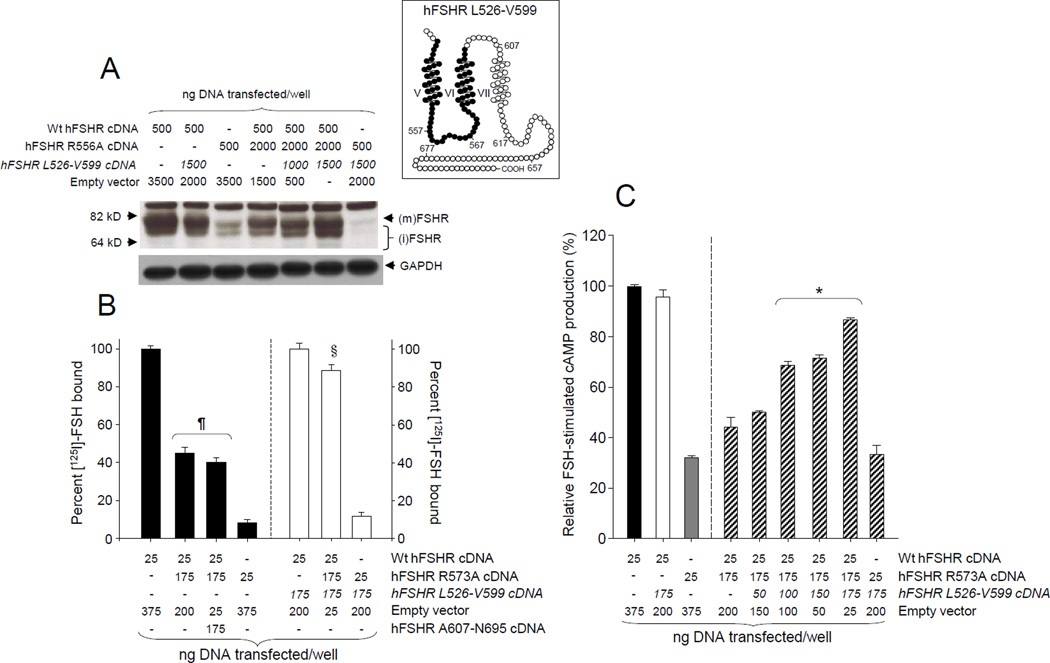
Figure 2.
Dominant negative effect of the laboratory-manufactured R573A mutant hFSHR on Wt hFSHR PM expression (A and B) and function (C), and effect of co-transfecting the Wt hFSHR L526-V599 fragment (inset, black circles) cDNA on the dominant negative effects of the mutant. (A) Representative autoradiogram from an immunoblot of the hFSHR present in protein extracts from HEK-293 cells transfected with the cDNAs shown at the top of the blot. The autoradiograph was overexposed in order to show the expression levels of the mutant receptor species and the immature forms of the hFSHR. (B) HEK-293 cells were co-transfected with the cDNAs shown at the bottom of the graph and specific [125I]-FSH binding was determined in the presence or absence of the L526-V599 fragment. Note that in contrast to the L526-V599 fragment, co-transfection with the hFSHR A607-N695 cDNA fragment (encoding the TM7 and the entire Ctail of the Wt receptor), did not affect expression of the Wt receptor co-transfected with the R573A mutant. (C) Maximal FSH-stimulated total cAMP accumulation in HEK-293 cells co-transfected with the cDNAs shown at the bottom of the graph; note that the L526-V599 fragment cDNA was co-transfected in increasing concentrations. The Wt and mutant hFSHR cDNAs were hosted by the pSG5 vector whereas the hFSHR fragments were in pcDNA3.1. The results shown in B and C are the mean ± SEM from three independent experiments. *p < 0.05 vs all other conditions; ¶p < 0.01 vs all other conditions; §p < 0.05 vs Wt + L526-V599 fragment + empty vector. (m)FSHR, mature form of the hFSHR; (i)FSHR, immature form of the hFSHR. Co-transfection of the mutant and Wt hFSHRs resulted in decreased PM expression and function of the mature form of the hFSHR as well as reduced specific [125I]-FSH binding. Co-transfection of the Wt and mutant FSHRs with the L526-V599 fragment led to almost complete functional recovery and PM expression of the hFSHR. Reproduced from Zariñán et al., (2010), with permission of Elsevier Ireland LTD.