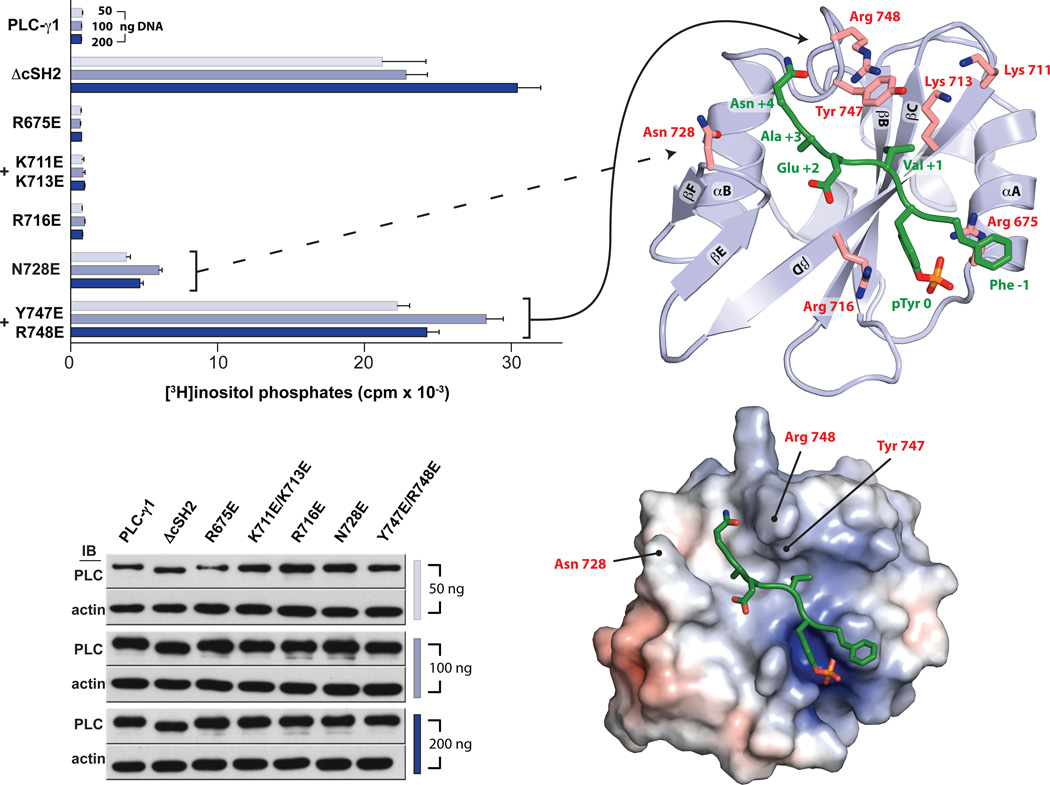
Fig. 3.
The auto-inhibitory interface within the cSH2 domain maps to the electropositive EF and BG loops. HEK293 cells were transfected with the indicated amounts of plasmids encoding either wild-type or mutant forms of PLC-γ1 prior to quantification of accumulated [3H]inositol phosphates (top left). Mutated residues are highlighted on the structure of the cSH2 domain (top right). Data are presented as the means ± S.E.M. of triplicate samples from a single experiment and are representative of three independent experiments. Expression of PLC-γ1 constructs confirmed by western blots of cell lysates (bottom left). Mutated residues that increase basal phospholipase activity are indicated on the solvent accessible surface of the cSH2 domain (bottom right) colored according to electrostatic potential calculated without bound peptide (red: −5 kT/e, blue: +5kT/e).