Scheme 1.
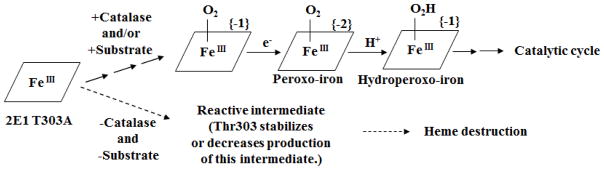
Possible pathway for the formation of the heme intermediates of P450 2E1 T303A when incubated with reductase and NADPH.
In the presence of catalase (i.e. the absence of hydrogen peroxide) and/or the presence of substrate, the top pathway is favored in which P450 2E1 T303A forms the previously described dioxygen intermediates (21, 50) leading to catalytic turnover. In this catalytic cycle there is: (i) a one-electron reduction of the P450 and binding of molecular oxygen to generate the oxyferrous P450, (ii) transfer of a second electron to form the peroxo-iron species, and (iii) addition of one proton to form the hydroperoxo-iron species which leads to further catalytic cycles. In the absence of both catalase (i.e., hydrogen peroxide is produced and remains in solution) and substrate, the bottom pathway becomes more favored and P450 2E1 T303A may form a reactive intermediate leading to heme-destruction. The reactive intermediate could be different from either the peroxo-iron or the hydroperoxo-iron species. We propose that the conserved threonine located in the active site of P450 2E1 either stabilizes this intermediate or decreases the production of the reactive heme-oxygen intermediate responsible for inactivation in order to decrease the rate of acceleration of inactivation that occurs in the presence of H2O2 under electron transfer conditions.
