Figure 2.
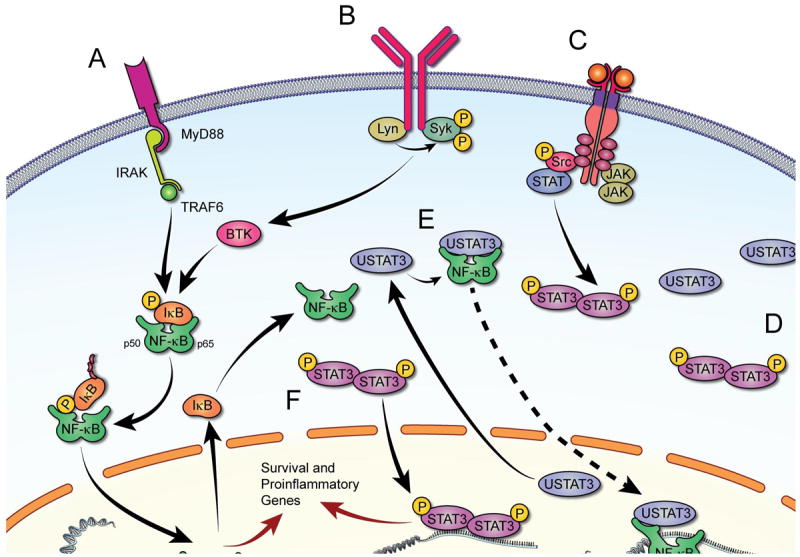
Signaling pathways activated in CLL cells. Upon attachment to their corresponding receptors (A, B and C) various extracellular factors induce signal transduction and activate transcription of inflammatory cytokines and chemokines. A. Ligands binding to TLR induce MYD88-mediated activation of NF-κB via phosphorylation and ubiquitination of IκB, enabling nuclear localization and DNA binding of NF-κB. B. BCR activation recruits BTK, which in turn phosphorylates IκB and activates NF-κB. C. Several ligands phosphorylate membrane-bound tyrosine kinase and non-tyrosine kinase receptors or recruit JAKs that transduce signaling by activating STAT3, ERK, and/or AKT (not shown). Phosphorylated (p) STAT3 forms heterodimers, shuttles to the nucleus, and activates transcription. D. In CLL, STAT3 is constitutively phosphorylated. Because STAT3 activates STAT3, high levels of unphosphorylated STAT3 (USTAT3) are present in CLL cells. E. USTAT3 binds NF-κB in competition with IκB; USTAT3 that shuttles freely in and out of the nucleus “carries” into the nucleus NF-κB that binds to DNA and activates transcription. F. Both pSTAT3 and NF-κB induce production of inflammatory cytokines that provide CLL cells with survival advantage.
