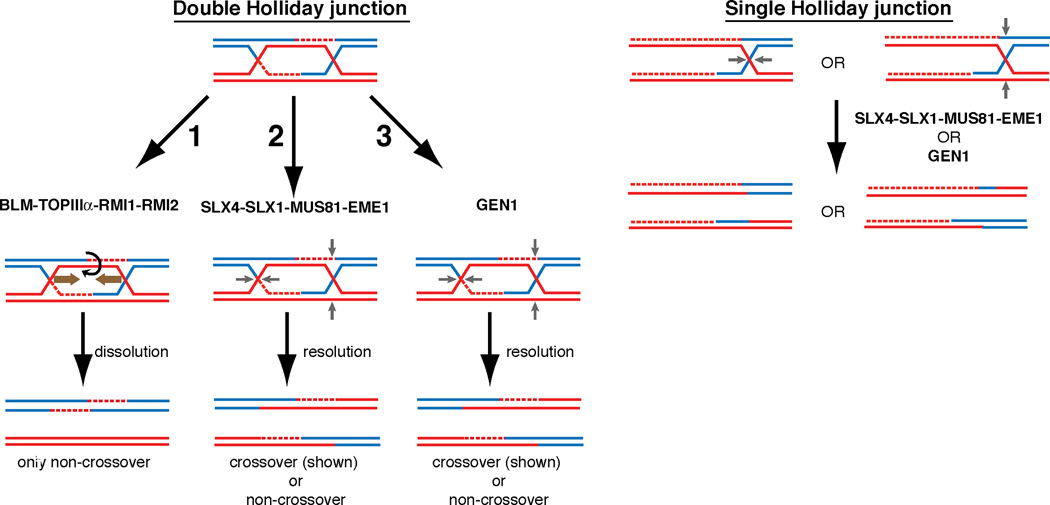
Figure 6. Model of Holliday junction processing in mitotically growing human cells.
(1) The BLM-TOP3α-RMI1-RMI2 Holliday junction dissolvase complex facilitates branch migration of dHJs towards one another and decatenation of the DNA strands without the use of structure specific endonucleases. This process yields entirely non-crossover reaction products. (2) SLX4 associated nucleases provide essential nucleolytic processing of dHJs that are not dissolved in the absence of the BLM complex. The strict requirement of both MUS81 and SLX1 interaction with SLX4 for the suppression of synthetic lethality in the absence of BLM as well as the increase of SCEs after MMC treatment or BLM depletion suggests that they together form an in vivo HJ resolvase. (3) GEN1 is able to process HJs but is unable to prevent the synthetic lethality of SLX4-BLM deficiency. Both SLX4-dependent or GEN1 mediated resolution of dHJs may yield both cross-over and non-crossover products. A single Holliday junction or another substrate unsuitable for BLM complex mediated activity requires SLX4-complexed nucleases or GEN1. GEN1-SLX4 synthetic lethality indicates that GEN1 activity is necessary in the setting of SLX4 deficiency even in the presence of the BLM complex. In this setting, SLX4-interacting MUS81-EME1 and SLX1 are again required for the suppression of GEN1-SLX4 synthetic lethality.