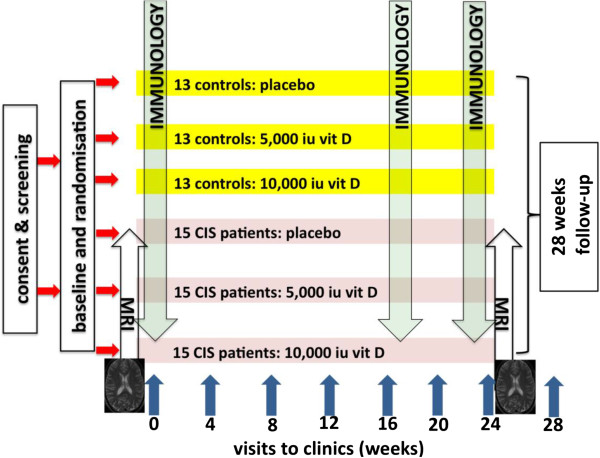
Figure 1.
Schematic diagram of study structure. There are two study groups of subjects: 39 healthy control participants and 45 patients with clinically isolated syndrome (CIS). Each of the two groups will be randomly allocated to one of three blinded treatments: placebo, vitamin D 5,000 IU, or vitamin D 10,000 IU daily. Study duration is 24 weeks with immunologic testing (primary endpoint) of all study participants at baseline, week 16, and week 24. There will be 4-weekly clinic visits to assess safety measures and clinical status. 4 weeks after completion of the study there will be a follow-up safety visit. Magnetic resonance imaging will be carried out at baseline and week 24 in the CIS population only.