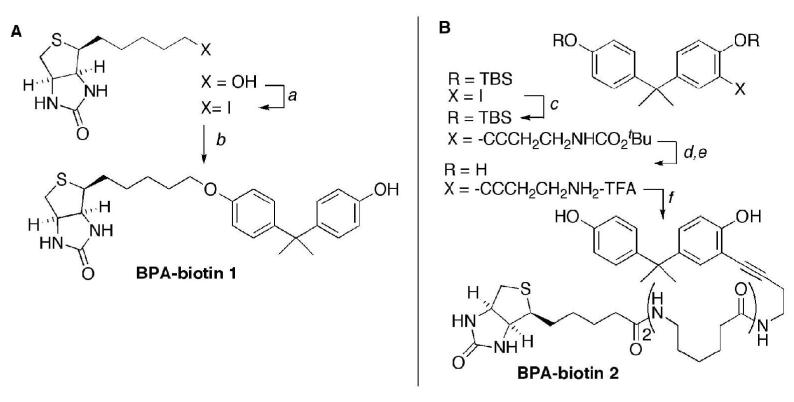
Figure 3. Synthesis of BPA-biotin affinity probes.
Panel A. The BPA-biotin derivative 1 was prepared by Williamson ether synthesis. The 5-hydroxy derivative was converted to 4-(5-iodopentyl)tetrahydro-1H-thieno[3,4-d]imidazol-2(3H)-one using I2, triphenylphosphine. Selective O-alkylation of BPA with the primary alkyl iodide gave the desired compound 1 as the major product.
Panel B. The BPA-biotin derivative 2 was prepared by sequential Sonogashira coupling and biotinylation. 4-(2-(4-Hydroxyphenyl)propan-2-yl)-2-iodophenol was protected as the bis-TBS ether using standard conditions, and was then coupled with tert-butyl but-3-ynylcarbamate to the alkyne product in excellent yield. The TBS protecting groups were removed with TBAF, and the tBoc group was cleaved with TFA to provide the corresponding ammonium salt. Biotinylation with biotin-L2-NHS in Et3N/DMF gave the desired compound 2. Both of the biotinylated probes 1 and 2 were purified by silica gel chromatography and structurally characterized by 1H- and 13C-NMR, and HPLC-MS.