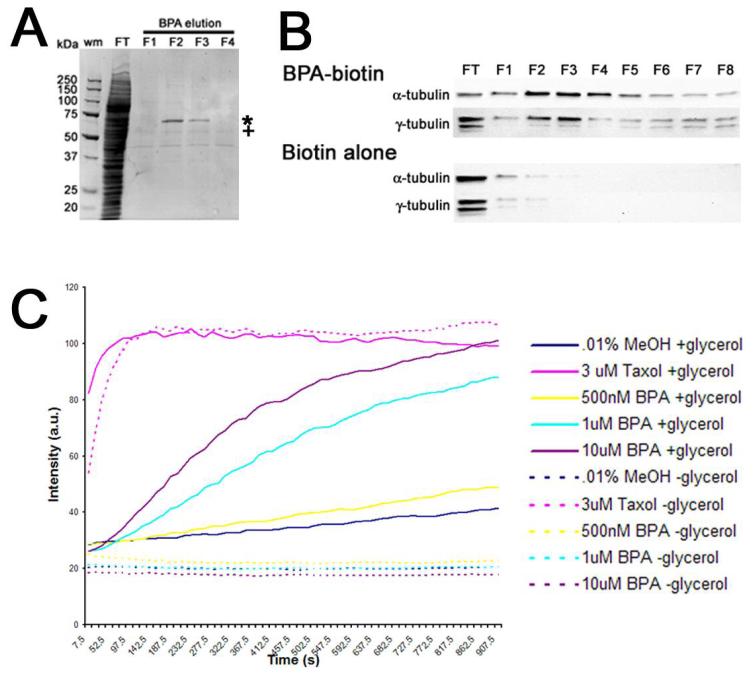
Figure 4. Bisphenol A binds tubulin and promotes microtubule polymerization.
Panel A. CSF-arrested Xenopus extracts (XE) were incubated with biotinylated BPA (compound 1), and protein complexes were fractionated over strepavidin beads, and following the collection of the flow-through (FT) fraction and washing with BRB80 buffer, bound proteins were eluted (F1-F4) with 210 μM BPA. Bound proteins were then resolved on a 4-15% SDS PAGE gradient gel. Two bands were chosen for analysis and sequencing (* and +), and the band denoted by (+) was identified by mass spectroscopy as α-/β-tubulin. Panel B. Western blot confirmation of α-tubulin and γ-tubulin elution from BPA-biotin 1 affinity matrices in fractions 1-8 (FT corresponds to the flow-through or unbound fraction). In contrast, extract incubated with biotin alone showed that neither α-tubulin or γ-tubulin associated with the affinity matrix. Panel C. Tubulin was polymerized in the presence of 20% glycerol (Solid lines) either in the presence of carrier alone (0.1% MeOH), 3 μM Taxol, or increased concentrations of BPA.A parallel set of assays were performed in the presence of low glycerol (2%), where microtubules polymerize poorly unless in the presence of an additional stabilizing reagent (dashed lines). Samples were assembled on ice, warmed to 37 °C and readings were acquired every 15 sec. Note that while taxol-induced polymerization was independent of glycerol, BPA-induced polymerization did not occur in the absence of glycerol.