Figure 1.
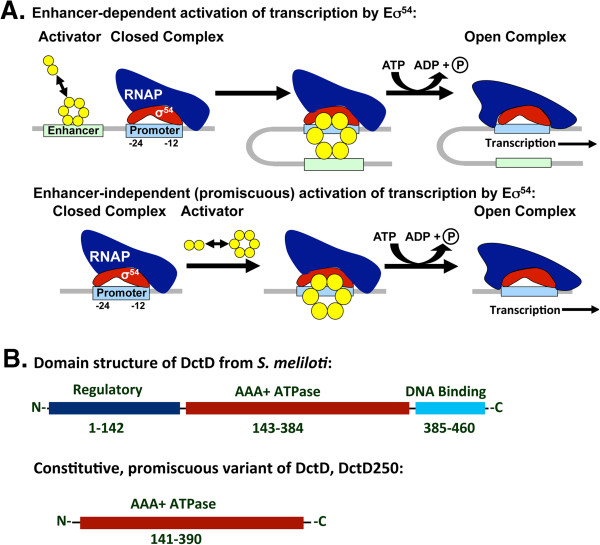
Activation of σ54-dependent transcription and activator structure. A) σ54 (red subunit) directs binding of the RNA polymerase (dark blue subunit) holoenzyme (Eσ54) to the -12, -24 promoter elements (light blue box). This closed complex is stable and cannot transition to open complex. In response to an environmental or cellular signal, the activator (bEBP; yellow dimers) oligomerizes. For most bEBPs, the oligomer binds to an enhancer (green box) 80 to 150 bp upstream of the promoter and DNA looping brings the activator in contact with σ54 in the Eσ54 closed complex. Hydrolysis of ATP by bEBP causes remodeling of Eσ54, which leads to open complex formation and transcription. There are a few bacteria with bEBPs that are missing the DNA binding domain; after oligomerization, these activators can bind to Eσ54 in closed complex with any promoter to stimulate open complex formation (promiscuous activation). B) The domain structure for the Sinorhizobium meliloti bEBP, DctD, is typical of most bEBPs. The amino-terminal regulatory domain (dark blue box) inhibits assembly of the bEBP oligomer until it interacts with an activation signal; the AAA+ ATPase domain (red box) mediates ATP binding and hydrolysis, as well as the protein-protein interactions between bEBPs (oligomerization) and between bEBP and σ54; the carboxyl-terminal DNA binding domain (aqua box) contains a helix-turn-helix motif for binding the enhancer. The truncated DctD variant, DctD250, is missing the regulatory and DNA binding domains, so that it is constitutively active and promiscuous in stimulating transcription from σ54-dependent promoters.