Abstract
The growth and function of tissues is critically dependent on their vascularization. Adipose tissue is capable of expanding many-fold during adulthood, therefore requiring the formation of new vasculature to supply growing and proliferating adipocytes. The expansion of the vasculature in adipose tissue occurs through angiogenesis, where new blood vessels develop from those pre-existing within the tissue. Inappropriate angiogenesis may underlie adipose tissue dysfunction in obesity, which in turn increases type-2 diabetes risk. In addition, genetic and developmental factors involved in vascular patterning may define the size and expandability of diverse adipose tissue depots, which are also associated with type-2 diabetes risk. Moreover, the adipose tissue vasculature appears to be the niche for pre-adipocyte precursors, and factors that affect angiogenesis may directly impact the generation of new adipocytes. Here we review recent advances on the basic mechanisms of angiogenesis, and on the role of angiogenesis in adipose tissue development and obesity. A substantial amount of data point to a deficit in adipose tissue angiogenesis as a contributing factor to insulin resistance and metabolic disease in obesity. These emerging findings support the concept of the adipose tissue vasculature as a source of new targets for metabolic disease therapies.
Keywords: adipocyte, endothelial, vascular, fat, depot, vascularization, weight gain, capillary, blood vessel, hypoxia
Introduction
The growth and function of all tissues and organs are critically dependent on their appropriate vascularization. Blood flow is important for providing the correct oxygen tension in individual tissues, for the delivery and removal of nutrients and waste products, and for the transit of cells involved in tissue immune surveillance. In addition to being critical for the health of individual tissues, blood flow delivers hormones and growth factors that insure the inter-tissue and inter-organ communication necessary for whole body homeostasis. Thus, the growth of any organ or tissue must be accompanied by parallel growth of its vascular network. Most of the growth of organs and tissues occurs during development, and their final size remains relatively constant through adulthood. In contrast, adipose tissue is unique in that it can expand many-fold, to comprise more than 40% of total body composition in obese individuals, defined as a body mass index of 30 or higher. The ability of adipose tissue to expand has clear evolutionary advantages, enabling survival in times of nutrient scarcity; however, concomitant with adipose tissue expansion are metabolic alterations that enhance risk of metabolic disease. The cellular and molecular mechanisms by which adipose tissue growth is coordinated with the expansion of its capillary network are unknown. As these mechanisms may underlie the basis for adipose tissue dysfunction in metabolic disease, they comprise a fertile and exciting area of research.
While the close association between weight gain and heightened risk of type 2 diabetes (T2DM) is well established, not all individuals with obesity become diabetic, and certain individuals become diabetic after very minor weight gain [1]. This paradox is explained by the large individual variation in the size and expandability of different adipose tissue depots in humans, as expansion of some depots is associated with increase risk, while expansion of others is associated with decreased risk [2]. Strikingly, each standard deviation (SD) increase in subcutaneous adipose tissue mass decreases the odds of insulin resistance by 48%. In contrast, each SD increase in visceral adipose tissue mass increases the odds of insulin resistance by 80% [3]. The protective effect of expandable subcutaneous fat depots during weight gain is likely to be due to their capacity to properly store excess calories in the form of triglycerides, thus preventing ectopic lipid deposition into muscle, liver and visceral fat depots. Such ectopic deposition and inappropriate lipid metabolism is thought to cause insulin resistance and result in a greatly increased risk of T2DM [4, 5]. Thus, understanding the specific mechanisms by which the subcutaneous adipose tissue expands is of particular interest, as these could provide new approaches for therapeutic intervention in metabolic disease. Several lines of evidence indicate that adipose tissue growth can be limited by its vascular supply [6-8], raising the possibility that the angiogenic potential of specific depots might be critical in limiting their maximal expandability. Testing this hypothesis will require a deep understanding of the basic mechanisms of vascularization in expanding adult tissues, and the specific regulatory factors that operate in different adipose tissue depots.
Basic molecular and cellular mechanisms of angiogenesis
The de-novo formation of blood vessels during the development of the embryo occurs through the process of vasculogenesis, in which mesoderm-derived precursors, called angioblasts, organize into the first primitive blood vessels. All further vessel growth during organ and tissue development, as well as during tissue repair in adult organism, takes place through the process of angiogenesis, in which new vessels sprout from pre-existing vasculature. It is likely therefore that the vascularization of adipose tissue depots in adults proceeds through angiogenic expansion of the existing vasculature, as has been shown to occur during the formation of fat pads from implanted cells [9]. The last few years have brought great insight into of the basic molecular and cellular mechanisms of angiogenesis [10-14], setting the stage for the identification of factors that regulate this process to fulfill tissue and developmental stage specific functions. Key insights into the cellular and molecular basis for angiogenesis, derived from experimental models such as the developing zebrafish embryo and the mouse retina, are very briefly summarized below.
The cardinal features of angiogenesis comprise the proliferation of endothelial cells, their directed migration through the extracellular matrix, the establishment of intercellular junctions, the formation of a lumen, the organization of perivascular supporting cells, the anastomosis with existing vessels, and the establishment of circulation. The cardinal initiating event is the stimulation of endothelial cell proliferation, which is mediated by the VEGF family of growth factors. These growth factors and their receptors have been established as master regulators of endothelial cell growth. In particular, VEGF-A, acting through the VEGFR2 (VEGF Receptor 2, also known as KDR in humans or Flk1 in mice) represents the most potent mitogenic and chemo attractant signal for endothelial cells. In response to VEGF-A gradients, endothelial cells divide, and acquire a specific phenotype (tip cell phenotype) characterized by the formation of branches and numerous filopodia, which extend towards the direction in which the endothelial cell migrates. The action of VEGFs and their receptors are critically controlled by the Notch signaling pathway, which modulates the responsiveness of endothelial cells to VEGF, and their subsequent specialization. Thus, tip cells are characterized by high levels of expression of Delta-like 4 (Dll4), which is a ligand for Notch. The stimulation of Notch signaling by Dll4 in the tip cell suppresses VEGF signaling in adjacent cell, resulting in the acquisition of a stalk-cell phenotype. The continuous dynamic interaction between VEGF, Notch and Dll4 results in the development of angiogenic sprouts. Newly formed sprouts are then stabilized by interactions with smooth muscle cells and pericytes, and become lumenized, through processes that appear to involve junctional trans-membrane proteins such as VE-cadherin, as well as matrix proteins which are broken down and reorganized dynamically during vessel growth [14]. Newly formed sprouts anastomose with existing vessels, thus extending tissue microcirculation.
While the basic steps of angiogenesis outlined above are expected to operate, it is likely that the vascular network of each organ and tissue will be established through key tissue-specific mechanisms. A prominent example is the regulation of angiogenesis in the central nervous system, where specific G-protein coupled receptors are uniquely expressed and play dominant roles in angiogenic vascularization of the developing brain [15, 16]. What mechanisms operate in adipose tissue, and how they modulate the basic steps of angiogenesis described above, are outstanding questions in adipose tissue biology.
Adipose tissue angiogenesis; what are the triggers?
One of the guiding questions for understanding angiogenic growth in adipose tissue is whether the mechanisms involved during embryonic and early postnatal development are similar to those involved in response to excess calorie consumption in adults. In both cases, two broad possibilities can be considered: First, angiogenic expansion may be triggered in response to signals emanating from proliferating and enlarging adipocytes. The second possibility is that angiogenic growth is triggered by developmental and/or metabolic signals, and parallels or precedes adipocyte proliferation and enlargement (Figure 1). These two possibilities are not mutually exclusive, and in all likelihood tissue expansion involves both local cues arising from expanding adipocytes, as well as distant cues reflecting the developmental and metabolic state of the whole organism.
Figure 1. Two possible models for the stimulation of angiogenesis during adipose tissue growth.
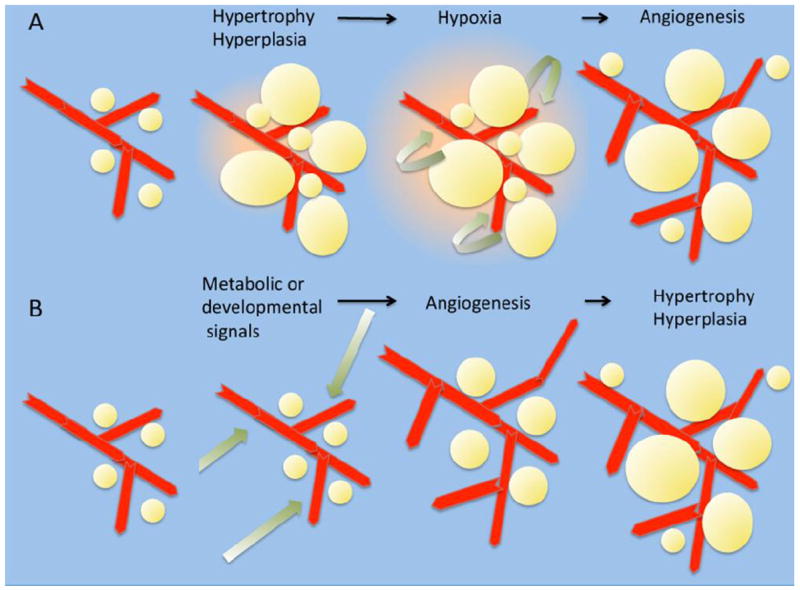
A. Increased caloie consumption results in adipocyte hypertrophy and hyperplasis, generating areas of tissue hypoxia. Hypoxia, and/or other factors released from the tissue stimulate angiogenesis. Angiogenesis results in mitigation of hypoxia and appropriate tissue architecture and function.
B. Increased calorie consumption results in systemic changes in trophic factors such as insulin, which directly stimulate angiogenesis within adipose tissue. Increased angiogenesis facilitates lipid storage in adipocytes and adipocyte hyperplasia. The simultaneous expansion of adipocytes and vasculature prevents development of hypoxia and metabolic stress.
The first option, in which vascular growth ensues secondarily to parenchymal growth is the canonical model for oncogenic vascularization [17-19]. In this model, the rapid growth of tumor cells and the formation of a tumor mass elicits regions of hypoxia. Hypoxia is sensed through multiple mechanisms, prominent amongst which is the inactivation of oxygen-dependent prolyl-hydroxylases. Inactivation of these enzymes results in the protection of HIF-1α from proteolytic degradation, allowing its dimerization with constitutively expressed HIF-1β to form the functional transcription factor HIF1. This transcription factor potently activates a program of hypoxia adaptation, which includes decreased transcription and translation and increased VEGF-A expression. The angiogenic expansion of the vasculature in response to VEGF-A enhances blood flow and relieves hypoxia, allowing further tumor growth. This model, in which tumor growth is absolutely dependent on stimulation of angiogenesis, forms the basis for the development and use of anti-angiogenic therapies in cancer [11].
Role of hypoxia in adipose tissue angiogenesis
The most relevant evidence consistent with a possible role for hypoxia in adipose tissue angiogenesis are the findings that adipose tissue in rodents becomes hypoxic in response to obesity that is rapidly induced by high fat diet (HFD). This finding has been documented repeatedly, using both chemical indicators of hypoxia, as well as direct monitoring of tissue oxygen tension using microelectrodes [20-22]. Using directly placed microelectodes, adipose tissue in obese humans has been found to be hypoxic [23]. Other findings consistent with a role for hypoxia are that the expression and secretion of pro-angiogenic factors by cultured adipocytes are strongly stimulated under low oxygen culture conditions [24]. These results suggest that, in a manner analogous to that occurring during tumor growth, adipose tissue hypoxia might be a driver for angiogenesis. However, the reported levels of hypoxia in human adipose tissue are relatively small, and one study actually finds increased oxygen tension in adipose tissue of obese subjects [25]. Moreover, it has been previously noted that expansion of adipose tissue in response to HFD is not accompanied a correspondingly increased blood flow [26]. Collectively, these results suggest that the response to hypoxia in adipose tissue may be insufficient to elicit sufficient compensatory angiogenic expansion.
A powerful, direct approach to defining the role of hypoxia in adipose tissue growth has been the tissue-specific overexpression and ablation of both HIF-1α and HIF-1β. Overexpression of a constitutively active form of HIF-1α in adipose tissue failed to induce a pro-angiogenic response; rather, it resulted in a fibrotic response and an increase in local inflammation [27]. Conversely, ablation of HIF-1α or HIF-1β (ARNT) in adipose tissue reduced fat formation, and protected from HFD-induced obesity and insulin resistance [28, 29]. Furthermore, anti-sense mediated depletion of HIF-1α in obese mice resulted in amelioration of HFD-induced insulin resistance [30], as did pharmaceutical inhibition of HIF-1α, as well as inducible expression of a dominant negative form [31]. Overall, these results are consistent with a model where adipose tissue hypoxia induces HIF-1α, which induces a fibrotic and inflammatory response rather than a compensatory pro-angiogenic response. Nevertheless, HIF-1α may be relevant for the growth and maintenance of brown adipose tissue, as expression of dominant-negative form of HIF-1α impairs thermogenesis and energy expenditure [32].
Role of VEGF in adipose tissue angiogenesis
In tumor angiogenesis, hypoxia-induced HIF1 stabilization activates VEGF transcription and secretion, which in turn stimulates angiogenesis. The induction of fibrosis and inflammation by HIF-1α in adipose tissue suggests that the stimulation of VEGF production may be controlled by different mechanisms, which are insufficiently activated during the rapid expansion induced by high-fat diets or hyperphagia. Consistent with this notion, transgenic overexpression of VEGF in adipose tissue results in increased vascularization, decreased inflammation, and amelioration of HFD-induced insulin resistance [33-35]. More strikingly, induced expression of VEGF-A in adipose tissue of animals previously rendered obese and insulin resistant reversed the established metabolic defects [33]. Expression of VEGF-A also caused the generation of adipocytes expressing UCP1, which are more similar to brown adipose tissue and have a higher metabolic rate, and result in lower weight gain under conditions of HFD [36].
Conversely, ablation of VEGF in adipose tissue resulted in hypo-perfused adipose tissue, which displayed higher levels of inflammatory markers even under normal chow diet. In response to HFD feeding adipose tissue from VEGF-ablated animals developed much greater inflammation compared to controls [33]. This greater inflammation was accompanied by adipocyte death, a net decrease in depot size, and marked deterioration of glucose tolerance and insulin sensitivity. This enhanced inflammatory phenotype is similar to that observed in animals overexpressing HIF-1α. In aggregate, these findings suggest a model where insufficient angiogenesis during high-fat diet leads to hypoxia, HIF-1α expression, inflammation and adipose tissue dysfunction (Figure 2). In addition, these studies clearly demonstrate that increased VEGF-A production and increased vascularization enables adipose tissue to adapt to rapid expansion caused by acutely increased caloric intake, and protects from the development of insulin resistance and glucose intolerance.
Figure 2. Different consequences of HIF expression in tumors and adipose tissue.
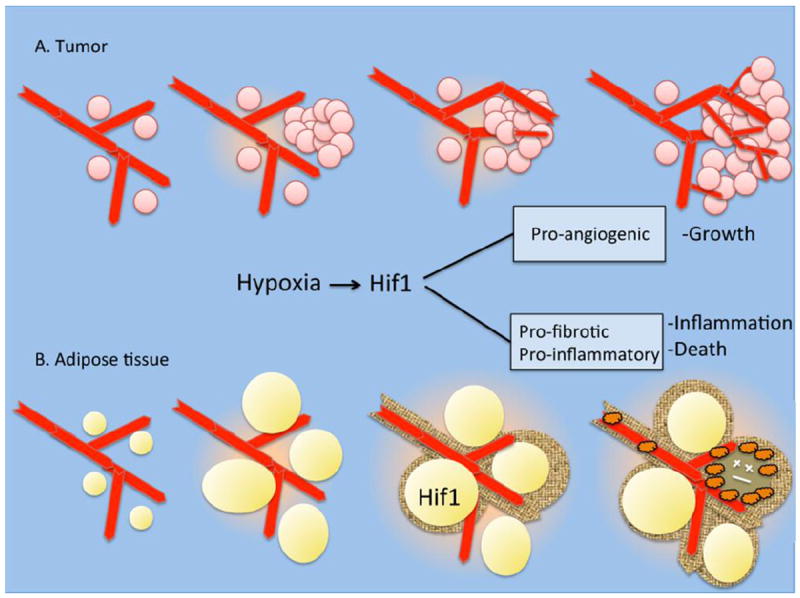
A. Rapid cell proliferation during tumor growth elicits hypoxia, which activates HIF1 signaling, VEGF production and angiogenesis. Increased vascularization allows the tumor to grow.
B. HIF1 expression in adipose tissue does not elicit VEGF production, but rather to a pro-fibrotic program, which is followed by inflammation, macrophage infiltration and cell death.
These results raise the questions: what mechanisms limit the expression of VEGF in adipose tissue, and do differences in VEGF production account for the variation in human adipose tissue expandability and subsequent protection from inflammation and metabolic dysfunction? Although some studies report decreased levels of VEGF gene expression in obese humans [23], others report higher levels of expression of VEGF-a in both subcutaneous and omental fat in obese compared to lean subjects, and a higher level in omental adipose tissue from obese insulin-sensitive compared to obese insulin-resistant individuals [37]. Unpublished results from our own group studying obese female subjects undergoing bariatric surgery are consistent with these later findings, suggesting that increased VEGF-A levels in adipose tissue may result in better vascularization and protection from inflammation and insulin resistance. Moreover, capillary density, as well as the capacity of human adipose tissue to produce new capillaries ex-vivo, is correlated with higher VEGF-A levels and increased insulin sensitivity in non-diabetic obese individuals.[38]. Thus, as in mouse models, increased levels of VEGF-A may facilitate healthy expansion of adipose tissue and protect from lipotoxicity and metabolic disease.
Hypoxia-independent mechanisms of VEGF production
The finding of beneficial effects of VEGF production and adipose tissue vascularization, as opposed to the deleterious effects of hypoxia signaling, suggest that mechanisms that increase VEGF production independently of hypoxia can confer a protective effect from metabolic risk. Several mechanisms can increase VEGF production independently from classical hypoxia signaling [39, 40]. These mechanisms are elicited in response to changes in cellular energy demands; for example, the co-activators PGC-1α and PGC-1β induce VEGF expression and angiogenesis in muscle in response to exercise[41-44]. These co-activators act on multiple transcription factors, including ERRs, PPARs and NRFs, to induce a program of adaptation to increased energy demand. The mechanism by which PGC-1α and PGC-1β stimulate VEGF production is complex, and may involve depletion of intracellular oxygen and stabilization of HIF-1α [45], as well as the direct co-activation of ERRα [43, 46]. Through a combination of these and possibly other mechanisms, PGC-1α and PGC-1β induce VEGF-A production in-vitro, and in-vivo result in enhanced muscle vascularization. Similar mechanisms may operate in brown adipose tissue, where cold induced capillary expansion is dependent on VEGF-A production. VEGF-A is induced in a hypoxia-independent manner [47, 48] through activation of PGC-1α in response to noradrenergic stimulation[47]. White adipose tissue contains detectable levels of PGC-1α [49, 50] and its adipose tissue-specific ablation results in insulin resistance [51]. Whether this phenotype is associated with decreased capillary density and angiogenic potential is an interesting question for future study.
Another metabolism-responsive mechanism reported to increase VEGF production is the activation of AMP-dependent protein kinase (AMPK)[52]. AMPK is a central integrator of energy balance, sensing the ratio of AMP/ATP within cells. AMPK is activated by conditions that lower cellular ATP, such as nutritional deprivation or hypoxia, and phosphorylates downstream targets that decrease energy utilization and enhance ATP production. Pharmacologically, AMPK activity can be stimulated in the absence of hypoxia by the AMP mimetic AICAR. AICAR stimulated VEGF mRNA and protein levels in C2C12 myotube cultures, and this effect was blocked by a dominant-negative AMPK [53]. In tumor cells, glucose deprivation caused an increase n VEGF expression, which was also blocked by expression of dominant-negative AMPK, and was not accompanied by changes in HIF-1α levels or transcriptional activity [52]. The mechanism by which AMPK activation results in increased VEGF levels appears to involve stabilization of VEGF mRNA, rather than transcriptional activation of the VEGF gene [52]. More recently, administration of AICAR increased VEGF mRNA in skeletal muscle of wild-type mice, but not in mice expressing dominant-negative AMPK [54]. As AMPK activity is stimulated in adipose tissue of subjects treated with metformin [55], it would be interesting to determine whether the insulin-sensitizing actions of this drug are associated with increased adipose tissue VEGF production.
Highly relevant to adipose tissue is the role of the peroxisome proliferator activated receptors (PPARs) in angiogenesis [56]. In particular, the role of PPARγ, a master regulator of adipocyte differentiation, is complex. While PPARγ activation inhibits proliferation of endothelial cells in-vitro [57-59], it has a net pro-angiogenic role in the context of adipose tissue, as determined by the increased capillary density and capillary sprouting capacity of adipose tissue obtained from both mice and humans treated with PPARγ agonist rosiglitazone [59, 60]. In addition, increased levels of VEGF are observed in adipose tissue of rodents treated with rosiglitazone [59, 61]. Furthermore, inhibition of PPARγ function in pre-adipocytes prevents not only the differentiation of adipocytes, but also the formation of vasculature in-vivo [62]. These results suggest that, while direct activation of endothelial cell PPARγ results in inhibition of angiogenesis, activation of PPARγ in the context of adipose tissue results in a net pro-angiogenic effect. This is likely to be due to the secretion of pro-angiogenic factors from adipocytes in response to PPARγ activation. Indeed, the ability of mature adipocytes to secrete potent pro-angiogenic factors, including VEGF, has been recognized for decades [63-67].
Factors other than VEGF involved in adipose tissue angiogenesis
Numerous pro and anti- angiogenic factors secreted by adipocytes are likely to control adipose tissue angiogenesis. A recent proteomic analysis of secreted proteins from adipose tissue depots suggest that as much as 50% of the adipose tissue secretome is comprised of proteins that have been implicated in angiogenesis [68], and various angiogenesis-related factors, shown in Table 1, are reported to be altered in response to obesity and HFD [38, 69-73]. One of the barriers in elucidating the specific role for these factors in adipose tissue angiogenesis is their pleiotropic expression and likely important roles in multiple tissues, requiring the generation of tissue-specific models to define their specific roles. Conversely, factors that play important roles in angiogenesis in other tissues may play minor roles in adipose tissue [74, 75]. Factors that are increased during adipocyte differentiation, are greatly overexpressed in adipose tissue, or are direct targets for PPARγ are thus of heightened relevance in this context.
Table 1.
Factors associated with adipose tissue angiogenesis.
| GENE/PROTEIN | ASSOCIATION WITH ADIPOSE TISSUE ANGIOGENESIS | REFERENCES |
|---|---|---|
| ANGPTL-4 (Angiopoietin- like 4) | Implicated in angiogenesis, lipid metabolism and glucose homeostasis; transcriptionally activated by PPARγ and hypoxia; highly expressed in adipose tissue, placenta and tumors. | [59, 79, 85, 91, 139] |
| APELIN | Highly expressed in adipose tissue; Ligand for G-protein coupled receptor APJ; required for vascular development in frog embryos; proangiogenic in human endothelial cells; up-regulated by hypoxia and during pregnancy and lactation. | [35, 71, 140-142] |
| PIGF/PLGF (Placental Growth Factor) | Interacts with VEGFR1; Inactivation impairs adipose tissue development. | [143, 144] |
| HGF (Hepatocyte Growth Factor) | Decreased formation of fat pads from 3T3- F442A cells with HGF knockdown. | [72] |
| FGF-1 (Fibrobalst Growth Factor-1) | Pathological adipose tissue vasculature in FGF-1 knockout mice under HFD. | [145] |
| SPARC/osteonectin/BM40 | Enriched in adipose tissue; Increased expression in obesity; promotes fibrosis. | [146-148] |
| Leptin | Produced exclusively by adipocytes; Induced vascularization in angiogenic assays; proangiogenic on human umbilical vein endothelial cells. | [67, 149, 150] |
| Adiponectin | Produced exclusively by adipocytes; chemo-attractant for endothelial progenitor cells; proangiogenic in human microvascular and umbilical vein endothelial cells. | [151-153] |
| Chemerin | Ligand for the G protein-coupled receptor CMKLR1; high level expression in mouse and human adipocytes; pro-angiogenic in human endothelial cells | [154, 155] |
| Ang-2 (Angiopoietin-2) | Increased in adipose tissue of obese mice; transcriptionally regulated by FOXC2 in adipose tissue. | [156, 157] |
Angiogenesis-related factors directly regulated in response to PPARγ activation, include angiopoietin-like 4 (ANGPTL4) [59, 76]. This factor was discovered simultaneously as a fasting-induced adipocyte factor [77], and as a direct target for PPARγ highly expressed in adipose issue and placenta [78]. ANGPTL4 inhibits lipoprotein lipase, and its expression is correlated with alterations in circulating lipids in mouse models and humans[79-82]. In parallel, ANGPTL4 has been found to be induced in tumors and normal tissues in response to hypoxia [83-87], and to have pro-angiogenic effects that affect tissue vascularization and wound healing [59, 88-91]. Overexpression of ANGPTL4 in obese mice ameliorates insulin resistance and glucose intolerance, even while inducing hyperlipidemia [92]. Whether the protective effect of increased ANGPTL4 results from increased adipose tissue vascularization or from inhibition of adipose tissue LPL is not known, but it is interesting to speculate that ANGPTL4 may be important for stimulating angiogenic expansion while preventing excess lipid accumulation in rapidly growing tissues.
In addition to factors secreted from adipocytes, non-adipocyte cells involved in tissue remodeling could induce adipose tissue angiogenesis. Infiltration of adipose tissue by cells of the immune system occurs rapidly in response to HFD in mice, and the visceral adipose depot of insulin-resistant humans is highly inflamed [93, 94]. While macrophage infiltration may result in inflammation-induced insulin resistance, these cells may also play a beneficial, trophic role, as has been suggested to occur in other tissues [95]. Indeed, a pro-angiogenic role for LYVE-1 positive macrophages in epididymal fat pad expansion has been shown [6], and a role for macrophages in stimulating tumor angiogenesis is extensively documented [96-100]. The angiogenic role of tissue macrophages has attributed to factors such as TNFα, IL-8, wnt and PDGF signaling [101-103], some of which also have important roles in adipocyte differentiation. Thus, deciphering the relative contribution of factors secreted by adipocytes and immune cells will be important for understanding the mechanisms of adipose tissue angiogenesis under diverse physiological conditions.
Developmental mechanisms of adipose tissue angiogenesis
In the paradigm of angiogenic growth described above the critical pro-angiogenic stimuli emanate from adipose cells undergoing hypoxia or energetic stress (Figure 1a). However, an alternative paradigm is one in which the growth of the vasculature precedes the growth of adipocytes, thus ensuring an adequate blood supply to the expanding tissue (Figure 1b). This paradigm functions during adipose tissue development in the embryo and early post-natal stages, and the possibility exists that it may also operate in adult tissue. A close association between adipocyte growth and the developing vasculature was noted as far back as 1870, where the emergence of adipocytes from well developed vascular networks led to the suggestion that a robust blood supply was essential for the development of adipose tissue. Extensive morphological analyses of the developing adipose tissue in pig and rodent embryo depots [8] strongly support the concept that the establishment of a capillary network precedes the emergence of adipocytes during embryonic development. Angiogenesis also precedes adipocyte formation during post-natal growth: In mouse epididymal fat, a dense vascular network in the tip of the fat pad expands rapidly during post-natal days 0-5, and new adipocytes arise from within the newly formed vessels [6, 104]. The activities of VEGF, VEGFR2, MMPs, and SDF-1 are essential, and administration of antibodies to VEGF inhibited angiogenesis as well as the emergence of adipocytes.
A functional requirement for vascular cells in adipocyte differentiation is also suggested by the experiments of Fukumura et al, mentioned above, in which implantation of pre adipocytes results in vigorous angiogenesis and the formation of fat pads [62]. The formation of the capillary network was dependent on the expression of PPARγ in the implanted cells, consistent with a major role of this transcription factors in regulating the expression of pro-angiogenic factors, as described above. Strikingly, inhibition of VEGF signaling blocked not only angiogenic growth but also the differentiation of the implanted pre-adipocytes, suggesting that direct interaction with endothelial cells, or other cells associated with blood vessels, may be crucial for the differentiation of pre-adipocytes in-vivo. These results are consistent with recent findings that the vasculature of adipose tissue is the niche for pre-adipocyte precursors [105, 106]. These precursors, labeled using lineage-specific PPARγ-driven markers, also express smooth muscle actin, PDGFR-β and NG2, which are characteristic markers of mural cells [105].
Cells within human adipose tissue capillaries formed ex-vivo also contain adipocyte precursors [107]; interestingly, these cells contained endothelial cell markers as well as large lipid droplets, glycogen particles and a displaced nucleus, all features of differentiated adipocytes. These results suggest that multi-potent cells with capacity for endothelial and/or adipocyte differentiation exist within the vessel wall. This notion is further supported by the finding that GFP expression driven by the Zfp423 promoter can be localized to cells within the adipose tissue vasculature, including a subset that express endothelial cell markers [108]. Because Zfp423 has been identified as a factor enriched in adipocyte precursors, these results are consistent with plasticity in the endothelial-adipose cell lineage, which has been suggested by others [109, 110]
The finding that pre-adipocyte precursors reside within the adipose tissue vasculature, and that inhibition of angiogenic growth inhibits pre-adipocyte differentiation, suggests that direct, cell-cell interactions that control angiogenic growth may also be involved in pre-adipocyte proliferation or differentiation in-vivo (Figure 3). Experiments in which adipose stromal cells co-cultured with endothelial cells display decreased differentiation, possibly due to activation of Wnt1 [111], are consistent with this possibility. In addition to Wnt signaling, Delta (dll4)-Notch signaling, which is crucial in the formation of new vessels, has also been associated with adipocyte differentiation [112-114].
Figure 3. Hypothetical model for coordination of angiogenesis and adipocyte proliferation.
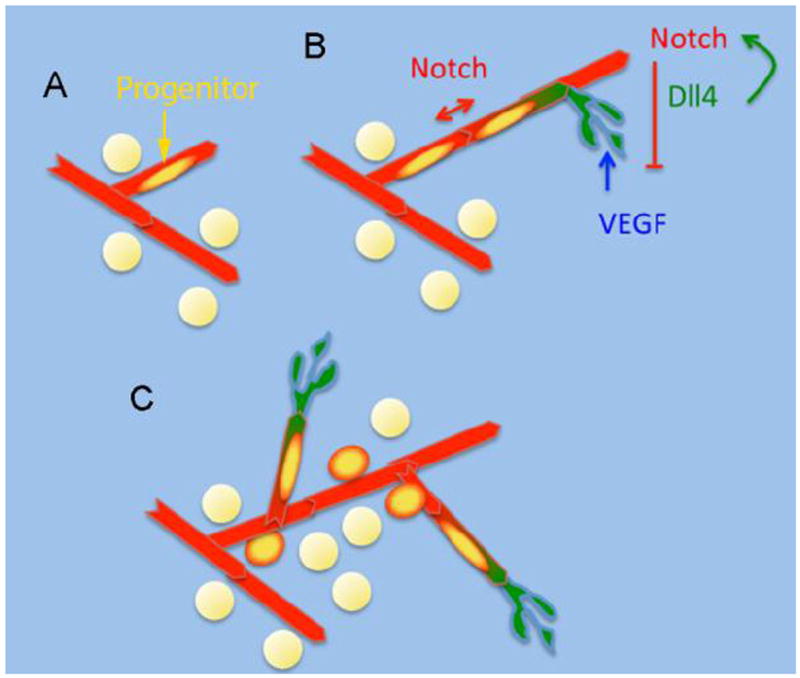
A. Quiescent multi-potent progenitors reside within the adipose tissue vasculature.
B. Endothelial cells are stimulated to proliferate by VEGF, resulting in the formation of tip cells (green) expressing Dll4, which suppresses VEGF signaling in adjacent cells via activation of Notch, forming new capillary sprouts. This is accompanied by proliferation of progenitors.
C. Progenitors differentiate into adipocytes and/or other cell types such as endothelial or mural cells.
Adipose tissue developmental genes and angiogenesis
Comprehensive analyses of gene expression in human adipose tissue reveal numerous differences related to functions such as lipolysis, fatty acid synthesis and inflammation among different depots. These differences in gene expression are consistent with the different functions of adipose tissue in different regions of the body, and with the variance in metabolic disease risk associated with expansion of specific depots [38]. In efforts to elucidate the developmental origins of these depots, and the mechanisms by which they attain specific functions, the expression of genes related to tissue patterning and embryonic development has been studied[115-117]. Importantly, major differences in expression of genes that determine body patterning have been found amongst different adipose depots. These include changes in expression of Hox genes, which are transcription factors that bind DNA through a 60 amino acid helix-turn-helix homeodomain, and control development along the anterior-posterior axis. A large proportion of the HOX gene network is active in adult white adipose tissue [115], and differences are seen in the levels of expression between whole visceral and subcutaneous adipose tissue from non-diabetic male humans [118]. In a comprehensive assessment of developmental gene expression between intra abdominal and subcutaneous depots from mouse and humans, highly significant differences in expression of Hox genes (HOXA5, HOXC8, HOXC9) were found consistently in both species [116]. More recently, differences amongst subcutaneous abdominal and gluteal depots of male and female humans have been reported [117]. Depot-associated variation included HOXA3, HOXA5, HOXB8, HOXC8, which were more highly expressed in abdominal adipose tissue, and HOXA10 and HOXC13, which were highly expressed in the gluteal depot in both genders. Importantly, variances seen in whole tissue are also observed when analyzing the stromovascular fraction, which encompasses a mixed population of endothelial cells, fibroblasts, white blood cells and in all likelihood adipocyte progenitors [116, 117].
The finding of significant differences in developmental gene expression in the stromovascular fraction of adipose tissue, together with the possible role for the vasculature as the niche for adipocyte precursors, raises the question of whether the role of these developmental genes is in fact exerted at the level of vascular development. Indeed, the vascular system is the first organ system developed during embryogenesis, and growth of tissues and organs is critically dependent on vascular expansion. Hox genes are important determinants of anterior-posterior development, but act at the cellular level by modulating functions such as cell adhesion, migration and cell cycle control, and are important in the process of angiogenic transcriptional regulation [119].
The developmental genes differentially expressed in adipose tissue depots include several which have been implicated in vascular patterning and endothelial function. Amongst these is HOXC9, which is expressed in different vascular beds, is negatively correlated with growth of human umbilical vein endothelial cells, and impairs vascular development in zebrafish upon endothelial-specific overexpression [119, 120]. Another is HOXA9, which is necessary for endothelial cell migration and tube formation [121], and has been reported to be essential for inflammatory activation of endothelial cells [121-123]. Both HOXC9 and HOXA9 are more highly expressed in subcutaneous compared to visceral adipose tissue in mice and lean humans, and HOXA9 is more highly expressed in gluteal compared to abdominal subcutaneous fat[116]. HOXA5 has also been implicated in vasculature development [124, 125], while correlating with levels of obesity and fat distribution in humans [117, 126]. Distinguishing the relationship between the role of these genes in vascular development and depot-specific adipose tissue growth is an exciting area for future research.
Impact of adipose tissue angiogenesis on obesity and insulin resistance
Several lines of evidence indicate that adipose tissue growth can be limited by its vascular supply [6-8], and that rapid adipose tissue expansion, such as that elicited by HFD in mice, is not adequately paralleled by growth of the capillary network, leading to hypoxia [20, 22, 23, 35, 127]; hypoxic stress in turn can promote inflammation [21] and insulin resistance. Indeed, recent experiments in mouse models of adipose tissue VEGF over and under expression provide direct and convincing evidence consistent with a critical role for angiogenesis in determining adipose tissue size, as well as impacting the metabolic sequelae associated with impaired adipose tissue growth. As noted above, adipose tissue VEGF-ablated animals display a net decrease in depot size, accompanied by inflammation and marked deterioration of glucose tolerance and insulin sensitivity, most evident in response to HFD. Conversely, overexpression of VEGF in adipose tissue results in increased vascularization, decreased inflammation, and amelioration of HFD-induced insulin resistance [33-35].
Several other instances have been described in which pro-and anti-angiogenic factors impact obesity and/or insulin resistance. For example, overexpression of ANGPTL4 in obese mice ameliorates insulin resistance and glucose intolerance, even while inducing hyperlipidemia [92]. In another example, mice lacking 11β-hydroxysteroid dehydrogenase type 1, which are resistant to HFD-induced metabolic disease even while displaying adipose tissue expansion, have higher adipose tissue vascular density, increased levels of pro-angiogenic factor mRNA levels, and increased secretion of pro-angiogenic factors [35].
While human studies are mostly correlations, existing data indicate that expansion of adipose tissue in adults is accompanied by induction of a pro-angiogenic gene expression profile [70]. Moreover, capillary density, as well as the capacity of human adipose tissue to produce new capillaries ex-vivo, is correlated with higher VEGF-A levels and increased insulin sensitivity in non-diabetic obese individuals [37, 38]. Thus, as in mice, increased VEGF-A levels in adipose tissue may result in better vascularization and protection from inflammation and insulin resistance.
Is adipose tissue angiogenesis a therapeutic target in Type-2 diabetes?
Initial recognition of a clear association between obesity and insulin resistance suggested that limiting the accretion of fat might also limit the risk of metabolic disease. Knowledge about the absolute requirement for angiogenesis in adipose tissue expansion led to early experiments in which anti-angiogenic compounds were used as possible anti-obesity and anti-diabetes therapeutic approaches [128, 129]. These approaches demonstrated decreased fat accumulation, however, their simultaneous effects to decrease food consumption confounded the interpretation of the beneficial results of anti-angiogenic therapy.
Subsequent demonstration that insulin resistance and disease risk most likely stem from insufficient fat storage and ensuing lipotoxicity and inflammation, rather than directly from increased fat accumulation [69, 130-132], logically has limited enthusiasm for approaches that focus on deceasing adipose tissue expansion without decreasing caloric input or enhancing energetic output. Indeed, the enhanced risk of diabetes accompanying lipodystrophy [133-135] and, conversely, the improved metabolic state in several models of increased adiposity [136, 137], further suggest that impairing adipose tissue growth is not a promising approach for ameliorating metabolic disease.
While anti-angiogenesis approaches in which appropriate adipose tissue vascularization and growth are compromised are unlikely to be of therapeutic benefit, the adipose tissue vasculature remains a promising site for identification of new therapeutic targets. Accumulating evidence for the adipose tissue vasculature as the niche for adipocyte progenitors raises the possibility of vasculature-targeted approaches to enhance the generation of healthy adipocytes, mitigating adipocyte stress and inflammation. Moreover, the vasculature is likely to be the niche for precursors cells giving rise to brite/beige adipocytes, which are metabolically active and may decrease metabolic disease risk [138, 139]. Better understanding of the mechanisms that induce adipose tissue angiogenesis, and of the role of the vasculature in adipose tissue function, will in all likelihood provide new and perhaps unexpected insights into ways adipose tissue biology can be targeted to improve human health.
Highlights.
Adipose tissue growth requires vascular expansion, which occurs through angiogenesis.
During development, the expansion of vasculature is required for the formation of adipose tissue depots.
In obesity, impaired vascularization is associated with adipose tissue malfunction and metabolic disease risk.
The mechanism that control adipose tissue angiogenesis are reviewed.
Acknowledgments
This work was funded by National Institutes of Health grant DK089101 to S. Corvera. The authors thank Dr. Michael Czech for valuable comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nakagami T, Qiao Q, Carstensen B, Nhr-Hansen C, Hu G, Tuomilehto J, Balkau B, Borch-Johnsen K. Age, body mass index and Type 2 diabetes-associations modified by ethnicity. Diabetologia. 2003;46:1063–1070. doi: 10.1007/s00125-003-1158-9. [DOI] [PubMed] [Google Scholar]
- 2.Preis SR, Massaro JM, Robins SJ, Hoffmann U, Vasan RS, Irlbeck T, Meigs JB, Sutherland P, D’Agostino RB, Sr, O’Donnell CJ, Fox CS. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–1760. doi: 10.1210/jc.2011-0615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochim Biophys Acta. 2010;1801:338–349. doi: 10.1016/j.bbalip.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Hardy OT, Czech MP, Corvera S. What causes the insulin resistance underlying obesity? Curr Opin Endocrinol Diabetes Obes. 2012;19:81–87. doi: 10.1097/MED.0b013e3283514e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho CH, Koh YJ, Han J, Sung HK, Jong Lee H, Morisada T, Schwendener RA, Brekken RA, Kang G, Oike Y, Choi TS, Suda T, Yoo OJ, Koh GY. Angiogenic role of LYVE-1-positive macrophages in adipose tissue. Circ Res. 2007;100:e47–57. doi: 10.1161/01.RES.0000259564.92792.93. [DOI] [PubMed] [Google Scholar]
- 7.Christiaens V, Lijnen HR. Angiogenesis and development of adipose tissue. Mol Cell Endocrinol. 2010;318:2–9. doi: 10.1016/j.mce.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Crandall DL, Hausman GJ, Kral JG. A review of the microcirculation of adipose tissue: anatomic, metabolic, and angiogenic perspectives. Microcirculation. 1997;4:211–232. doi: 10.3109/10739689709146786. [DOI] [PubMed] [Google Scholar]
- 9.Neels JG, Thinnes T, Loskutoff DJ. Angiogenesis in an in vivo model of adipose tissue development. Faseb J. 2004;18:983–985. doi: 10.1096/fj.03-1101fje. [DOI] [PubMed] [Google Scholar]
- 10.Eilken HM, Adams RH. Dynamics of endothelial cell behavior in sprouting angiogenesis. Curr Opin Cell Biol. 2010;22:617–625. doi: 10.1016/j.ceb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 11.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung AS, Ferrara N. Developmental and pathological angiogenesis. Annu Rev Cell Dev Biol. 2011;27:563–584. doi: 10.1146/annurev-cellbio-092910-154002. [DOI] [PubMed] [Google Scholar]
- 13.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 14.Senger DR, Davis GE. Angiogenesis. Cold Spring Harb Perspect Biol. 2011;3:a005090. doi: 10.1101/cshperspect.a005090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson KD, Pan L, Yang XM, Hughes VC, Walls JR, Dominguez MG, Simmons MV, Burfeind P, Xue Y, Wei Y, Macdonald LE, Thurston G, Daly C, Lin HC, Economides AN, Valenzuela DM, Murphy AJ, Yancopoulos GD, Gale NW. Angiogenic sprouting into neural tissue requires Gpr124, an orphan G protein-coupled receptor. Proc Natl Acad Sci U S A. 2011;108:2807–2812. doi: 10.1073/pnas.1019761108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, Su H, Fruttiger M, Young WL, Heilshorn SC, Kuo CJ. Essential regulation of CNS angiogenesis by the orphan G protein-coupled receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nat Med. 2011;17:1359–1370. doi: 10.1038/nm.2537. [DOI] [PubMed] [Google Scholar]
- 18.Germain S, Monnot C, Muller L, Eichmann A. Hypoxia-driven angiogenesis: role of tip cells and extracellular matrix scaffolding. Current opinion in hematology. 2010;17:245–251. doi: 10.1097/MOH.0b013e32833865b9. [DOI] [PubMed] [Google Scholar]
- 19.Cao Y, Arbiser J, D’Amato RJ, D’Amore PA, Ingber DE, Kerbel R, Klagsbrun M, Lim S, Moses MA, Zetter B, Dvorak H, Langer R. Forty-year journey of angiogenesis translational research. Sci Transl Med. 2011;3:114rv113. doi: 10.1126/scitranslmed.3003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rausch ME, Weisberg S, Vardhana P, Tortoriello DV. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int J Obes (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- 21.Ye J, Gao Z, Yin J, He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am J Physiol Endocrinol Metab. 2007;293:E1118–1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- 22.Hosogai N, Fukuhara A, Oshima K, Miyata Y, Tanaka S, Segawa K, Furukawa S, Tochino Y, Komuro R, Matsuda M, Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 23.Pasarica M, Sereda OR, Redman LM, Albarado DC, Hymel DT, Roan LE, Rood JC, Burk DH, Smith SR. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lolmede K, Durand de Saint Front V, Galitzky J, Lafontan M, Bouloumie A. Effects of hypoxia on the expression of proangiogenic factors in differentiated 3T3-F442A adipocytes. Int J Obes Relat Metab Disord. 2003;27:1187–1195. doi: 10.1038/sj.ijo.0802407. [DOI] [PubMed] [Google Scholar]
- 25.Goossens GH, Bizzarri A, Venteclef N, Essers Y, Cleutjens JP, Konings E, Jocken JW, Cajlakovic M, Ribitsch V, Clement K, Blaak EE. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- 26.Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- 27.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484–2495. doi: 10.2337/db11-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee KY, Gesta S, Boucher J, Wang XL, Kahn CR. The differential role of Hif1beta/Arnt and the hypoxic response in adipose function, fibrosis, and inflammation. Cell Metab. 2011;14:491–503. doi: 10.1016/j.cmet.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1alpha. PLoS One. 2012;7:e46562. doi: 10.1371/journal.pone.0046562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1alpha ameliorates adipose tissue dysfunction. Molecular and cellular biology. 2013;33:904–917. doi: 10.1128/MCB.00951-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X, Lam KS, Ye H, Chung SK, Zhou M, Wang Y, Xu A. Adipose tissue-specific inhibition of hypoxia-inducible factor 1{alpha} induces obesity and glucose intolerance by impeding energy expenditure in mice. J Biol Chem. 2010;285:32869–32877. doi: 10.1074/jbc.M110.135509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sung HK, Doh KO, Son JE, Park JG, Bae Y, Choi S, Nelson SM, Cowling R, Nagy K, Michael IP, Koh GY, Adamson SL, Pawson T, Nagy A. Adipose Vascular Endothelial Growth Factor Regulates Metabolic Homeostasis through Angiogenesis. Cell Metab. 2013;17:61–72. doi: 10.1016/j.cmet.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Wree A, Mayer A, Westphal S, Beilfuss A, Canbay A, Schick RR, Gerken G, Vaupel P. Adipokine expression in brown and white adipocytes in response to hypoxia. J Endocrinol Invest. 2012;35:522–527. doi: 10.3275/7964. [DOI] [PubMed] [Google Scholar]
- 35.Michailidou Z, Turban S, Miller E, Zou X, Schrader J, Ratcliffe PJ, Hadoke PW, Walker BR, Iredale JP, Morton NM, Seckl JR. Increased angiogenesis protects against adipose hypoxia and fibrosis in metabolic disease-resistant 11beta-hydroxysteroid dehydrogenase type 1 (HSD1)-deficient mice. J Biol Chem. 2012;287:4188–4197. doi: 10.1074/jbc.M111.259325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun K, Wernstedt Asterholm I, Kusminski CM, Bueno AC, Wang ZV, Pollard JW, Brekken RA, Scherer PE. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc Natl Acad Sci U S A. 2012;109:5874–5879. doi: 10.1073/pnas.1200447109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tinahones FJ, Coin-Araguez L, Mayas MD, Garcia-Fuentes E, Hurtado-Del-Pozo C, Vendrell J, Cardona F, Calvo RM, Obregon MJ, El Bekay R. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC Physiol. 2012;12:4. doi: 10.1186/1472-6793-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, Tran KV, Straubhaar J, Nicoloro S, Czech MP, Thompson M, Perugini RA, Corvera S. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fraisl P, Mazzone M, Schmidt T, Carmeliet P. Regulation of angiogenesis by oxygen and metabolism. Dev Cell. 2009;16:167–179. doi: 10.1016/j.devcel.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 40.Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P. Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet. 2008;40:170–180. doi: 10.1038/ng.2007.62. [DOI] [PubMed] [Google Scholar]
- 41.Arany Z, Foo SY, Ma Y, Ruas JL, Bommi-Reddy A, Girnun G, Cooper M, Laznik D, Chinsomboon J, Rangwala SM, Baek KH, Rosenzweig A, Spiegelman BM. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1alpha. Nature. 2008;451:1008–1012. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 42.Chinsomboon J, Ruas J, Gupta RK, Thom R, Shoag J, Rowe GC, Sawada N, Raghuram S, Arany Z. The transcriptional coactivator PGC-1alpha mediates exercise-induced angiogenesis in skeletal muscle. Proc Natl Acad Sci U S A. 2009;106:21401–21406. doi: 10.1073/pnas.0909131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rowe GC, Jang C, Patten IS, Arany Z. PGC-1beta regulates angiogenesis in skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E155–163. doi: 10.1152/ajpendo.00681.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H. PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab. 2009;297:E92–103. doi: 10.1152/ajpendo.00076.2009. [DOI] [PubMed] [Google Scholar]
- 45.O’Hagan KA, Cocchiglia S, Zhdanov AV, Tambuwala MM, Cummins EP, Monfared M, Agbor TA, Garvey JF, Papkovsky DB, Taylor CT, Allan BB. PGC-1alpha is coupled to HIF-1alpha-dependent gene expression by increasing mitochondrial oxygen consumption in skeletal muscle cells. Proc Natl Acad Sci U S A. 2009;106:2188–2193. doi: 10.1073/pnas.0808801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Auxenfans C, Lequeux C, Perrusel E, Mojallal A, Kinikoglu B, Damour O. Adipose-derived stem cells (ASCs) as a source of endothelial cells in the reconstruction of endothelialized skin equivalents. J Tissue Eng Regen Med. 2012;6:512–518. doi: 10.1002/term.454. [DOI] [PubMed] [Google Scholar]
- 47.Fredriksson JM, Nikami H, Nedergaard J. Cold-induced expression of the VEGF gene in brown adipose tissue is independent of thermogenic oxygen consumption. FEBS Lett. 2005;579:5680–5684. doi: 10.1016/j.febslet.2005.09.044. [DOI] [PubMed] [Google Scholar]
- 48.Xue Y, Petrovic N, Cao R, Larsson O, Lim S, Chen S, Feldmann HM, Liang Z, Zhu Z, Nedergaard J, Cannon B, Cao Y. Hypoxia-independent angiogenesis in adipose tissues during cold acclimation. Cell Metab. 2009;9:99–109. doi: 10.1016/j.cmet.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wilson-Fritch L, Nicoloro S, Chouinard M, Lazar MA, Chui PC, Leszyk J, Straubhaar J, Czech MP, Corvera S. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J Clin Invest. 2004;114:1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kleiner S, Mepani RJ, Laznik D, Ye L, Jurczak MJ, Jornayvaz FR, Estall JL, Chatterjee Bhowmick D, Shulman GI, Spiegelman BM. Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proc Natl Acad Sci U S A. 2012;109:9635–9640. doi: 10.1073/pnas.1207287109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yun H, Lee M, Kim SS, Ha J. Glucose deprivation increases mRNA stability of vascular endothelial growth factor through activation of AMP-activated protein kinase in DU145 prostate carcinoma. J Biol Chem. 2005;280:9963–9972. doi: 10.1074/jbc.M412994200. [DOI] [PubMed] [Google Scholar]
- 53.Ouchi N, Shibata R, Walsh K. AMP-activated protein kinase signaling stimulates VEGF expression and angiogenesis in skeletal muscle. Circ Res. 2005;96:838–846. doi: 10.1161/01.RES.0000163633.10240.3b. [DOI] [PubMed] [Google Scholar]
- 54.Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. The Journal of physiology. 2008;586:6021–6035. doi: 10.1113/jphysiol.2008.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boyle JG, Logan PJ, Jones GC, Small M, Sattar N, Connell JM, Cleland SJ, Salt IP. AMP-activated protein kinase is activated in adipose tissue of individuals with type 2 diabetes treated with metformin: a randomised glycaemia-controlled crossover study. Diabetologia. 2011;54:1799–1809. doi: 10.1007/s00125-011-2126-4. [DOI] [PubMed] [Google Scholar]
- 56.Bishop-Bailey D. PPARs and angiogenesis. Biochem Soc Trans. 2011;39:1601–1605. doi: 10.1042/BST20110643. [DOI] [PubMed] [Google Scholar]
- 57.Kim KY, Ahn JH, Cheon HG. Anti-angiogenic action of PPARgamma ligand in human umbilical vein endothelial cells is mediated by PTEN upregulation and VEGFR-2 downregulation. Mol Cell Biochem. 2011;358:375–385. doi: 10.1007/s11010-011-0989-9. [DOI] [PubMed] [Google Scholar]
- 58.Sarayba MA, Li L, Tungsiripat T, Liu NH, Sweet PM, Patel AJ, Osann KE, Chittiboyina A, Benson SC, Pershadsingh HA, Chuck RS. Inhibition of corneal neovascularization by a peroxisome proliferator-activated receptor-gamma ligand. Exp Eye Res. 2005;80:435–442. doi: 10.1016/j.exer.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPARgamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295:E1056–1064. doi: 10.1152/ajpendo.90345.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gealekman O, Guseva N, Gurav K, Gusev A, Hartigan C, Thompson M, Malkani S, Corvera S. Effect of rosiglitazone on capillary density and angiogenesis in adipose tissue of normoglycaemic humans in a randomised controlled trial. Diabetologia. 2012;55:2794–2799. doi: 10.1007/s00125-012-2658-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sotiropoulos KB, Clermont A, Yasuda Y, Rask-Madsen C, Mastumoto M, Takahashi J, Della Vecchia K, Kondo T, Aiello LP, King GL. Adipose-specific effect of rosiglitazone on vascular permeability and protein kinase C activation: novel mechanism for PPARgamma agonist’s effects on edema and weight gain. Faseb J. 2006;20:1203–1205. doi: 10.1096/fj.05-4617fje. [DOI] [PubMed] [Google Scholar]
- 62.Fukumura D, Ushiyama A, Duda DG, Xu L, Tam J, Krishna V, Chatterjee K, Garkavtsev I, Jain RK. Paracrine regulation of angiogenesis and adipocyte differentiation during in vivo adipogenesis. Circ Res. 2003;93:e88–97. doi: 10.1161/01.RES.0000099243.20096.FA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Castellot JJ, Jr, Karnovsky MJ, Spiegelman BM. Differentiation-dependent stimulation of neovascularization and endothelial cell chemotaxis by 3T3 adipocytes. Proc Natl Acad Sci U S A. 1982;79:5597–5601. doi: 10.1073/pnas.79.18.5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Folkman J. Angiogenesis: initiation and control. Ann N Y Acad Sci. 1982;401:212–227. doi: 10.1111/j.1749-6632.1982.tb25720.x. [DOI] [PubMed] [Google Scholar]
- 65.Silverman KJ, Lund DP, Zetter BR, Lainey LL, Shahood JA, Freiman DG, Folkman J, Barger AC. Angiogenic activity of adipose tissue. Biochem Biophys Res Commun. 1988;153:347–352. doi: 10.1016/s0006-291x(88)81229-4. [DOI] [PubMed] [Google Scholar]
- 66.Zhang QX, Magovern CJ, Mack CA, Budenbender KT, Ko W, Rosengart TK. Vascular endothelial growth factor is the major angiogenic factor in omentum: mechanism of the omentum-mediated angiogenesis. J Surg Res. 1997;67:147–154. doi: 10.1006/jsre.1996.4983. [DOI] [PubMed] [Google Scholar]
- 67.Bouloumie A, Drexler HC, Lafontan M, Busse R. Leptin, the product of Ob gene, promotes angiogenesis. Circ Res. 1998;83:1059–1066. doi: 10.1161/01.res.83.10.1059. [DOI] [PubMed] [Google Scholar]
- 68.Hocking SL, Wu LE, Guilhaus M, Chisholm DJ, James DE. Intrinsic depot-specific differences in the secretome of adipose tissue, preadipocytes, and adipose tissue-derived microvascular endothelial cells. Diabetes. 2010;59:3008–3016. doi: 10.2337/db10-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lemoine AY, Ledoux S, Queguiner I, Calderari S, Mechler C, Msika S, Corvol P, Larger E. Link between adipose tissue angiogenesis and fat accumulation in severely obese subjects. J Clin Endocrinol Metab. 2012;97:E775–780. doi: 10.1210/jc.2011-2649. [DOI] [PubMed] [Google Scholar]
- 70.Alligier M, Meugnier E, Debard C, Lambert-Porcheron S, Chanseaume E, Sothier M, Loizon E, Hssain AA, Brozek J, Scoazec JY, Morio B, Vidal H, Laville M. Subcutaneous adipose tissue remodeling during the initial phase of weight gain induced by overfeeding in humans. J Clin Endocrinol Metab. 2012;97:E183–192. doi: 10.1210/jc.2011-2314. [DOI] [PubMed] [Google Scholar]
- 71.Kunduzova O, Alet N, Delesque-Touchard N, Millet L, Castan-Laurell I, Muller C, Dray C, Schaeffer P, Herault JP, Savi P, Bono F, Valet P. Apelin/APJ signaling system: a potential link between adipose tissue and endothelial angiogenic processes. Faseb J. 2008;22:4146–4153. doi: 10.1096/fj.07-104018. [DOI] [PubMed] [Google Scholar]
- 72.Bell LN, Cai L, Johnstone BH, Traktuev DO, March KL, Considine RV. A central role for hepatocyte growth factor in adipose tissue angiogenesis. Am J Physiol Endocrinol Metab. 2008;294:E336–344. doi: 10.1152/ajpendo.00272.2007. [DOI] [PubMed] [Google Scholar]
- 73.Scroyen I, Jacobs F, Cosemans L, De Geest B, Lijnen HR. Blood vessel density in de novo formed adipose tissue is decreased upon overexpression of TIMP-1. Obesity (Silver Spring) 2010;18:638–640. doi: 10.1038/oby.2009.279. [DOI] [PubMed] [Google Scholar]
- 74.Van Hul M, Frederix L, Lijnen HR. Role of thrombospondin-2 in murine adipose tissue angiogenesis and development. Obesity (Silver Spring) 2012;20:1757–1762. doi: 10.1038/oby.2011.260. [DOI] [PubMed] [Google Scholar]
- 75.Lijnen HR, Frederix L, Van Hoef B, Dewerchin M. Deficiency of vascular endothelial growth factor-D does not affect murine adipose tissue development. Biochemical and biophysical research communications. 2009;378:255–258. doi: 10.1016/j.bbrc.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 76.Tian L, Zhou J, Casimiro MC, Liang B, Ojeifo JO, Wang M, Hyslop T, Wang C, Pestell RG. Activating peroxisome proliferator-activated receptor gamma mutant promotes tumor growth in vivo by enhancing angiogenesis. Cancer Res. 2009;69:9236–9244. doi: 10.1158/0008-5472.CAN-09-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, Gonzalez FJ, Desvergne B, Wahli W. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J Biol Chem. 2000;275:28488–28493. doi: 10.1074/jbc.M004029200. [DOI] [PubMed] [Google Scholar]
- 78.Yoon JC, Chickering TW, Rosen ED, Dussault B, Qin Y, Soukas A, Friedman JM, Holmes WE, Spiegelman BM. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Molecular and cellular biology. 2000;20:5343–5349. doi: 10.1128/mcb.20.14.5343-5349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lichtenstein L, Mattijssen F, de Wit NJ, Georgiadi A, Hooiveld GJ, van der Meer R, He Y, Qi L, Koster A, Tamsma JT, Tan NS, Muller M, Kersten S. Angptl4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010;12:580–592. doi: 10.1016/j.cmet.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, Cohen JC. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nature genetics. 2007;39:513–516. doi: 10.1038/ng1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Desai U, Lee EC, Chung K, Gao C, Gay J, Key B, Hansen G, Machajewski D, Platt KA, Sands AT, Schneider M, Van Sligtenhorst I, Suwanichkul A, Vogel P, Wilganowski N, Wingert J, Zambrowicz BP, Landes G, Powell DR. Lipid-lowering effects of anti-angiopoietin-like 4 antibody recapitulate the lipid phenotype found in angiopoietin-like 4 knockout mice. Proc Natl Acad Sci U S A. 2007;104:11766–11771. doi: 10.1073/pnas.0705041104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yoshida K, Shimizugawa T, Ono M, Furukawa H. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J Lipid Res. 2002;43:1770–1772. doi: 10.1194/jlr.c200010-jlr200. [DOI] [PubMed] [Google Scholar]
- 83.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY. Chronic Intermittent Hypoxia Induces Atherosclerosis via Activation of Adipose Angiopoietin-like 4. Am J Respir Crit Care Med. 2013 doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mazzatti D, Lim FL, O’Hara A, Wood IS, Trayhurn P. A microarray analysis of the hypoxia-induced modulation of gene expression in human adipocytes. Arch Physiol Biochem. 2012;118:112–120. doi: 10.3109/13813455.2012.654611. [DOI] [PubMed] [Google Scholar]
- 85.Gonzalez-Muniesa P, de Oliveira C, Perez de Heredia F, Thompson MP, Trayhurn P. Fatty acids and hypoxia stimulate the expression and secretion of the adipokine ANGPTL4 (angiopoietin-like protein 4/fasting-induced adipose factor) by human adipocytes. J Nutrigenet Nutrigenomics. 2011;4:146–153. doi: 10.1159/000327774. [DOI] [PubMed] [Google Scholar]
- 86.Murata M, Yudo K, Nakamura H, Chiba J, Okamoto K, Suematsu N, Nishioka K, Beppu M, Inoue K, Kato T, Masuko K. Hypoxia upregulates the expression of angiopoietin-like-4 in human articular chondrocytes: role of angiopoietin-like-4 in the expression of matrix metalloproteinases and cartilage degradation. J Orthop Res. 2009;27:50–57. doi: 10.1002/jor.20703. [DOI] [PubMed] [Google Scholar]
- 87.Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J, Philippe J, Sibony M, Gasc JM, Corvol P, Germain S. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162:1521–1528. doi: 10.1016/S0002-9440(10)64285-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yokouchi H, Eto K, Nishimura W, Takeda N, Kaburagi Y, Yamamoto S, Yasuda K. Angiopoietin-like protein 4 (ANGPTL4) is induced by high glucose in retinal pigment epithelial cells and exhibits potent angiogenic activity on retinal endothelial cells. Acta ophthalmologica. 2013 doi: 10.1111/aos.12097. [DOI] [PubMed] [Google Scholar]
- 89.Perdiguero EG, Galaup A, Durand M, Teillon J, Philippe J, Valenzuela DM, Murphy AJ, Yancopoulos GD, Thurston G, Germain S. Alteration of developmental and pathological retinal angiogenesis in angptl4-deficient mice. J Biol Chem. 2011;286:36841–36851. doi: 10.1074/jbc.M111.220061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mikhak B, Weinsheimer S, Pawlikowska L, Poon A, Kwok PY, Lawton MT, Chen Y, Zaroff JG, Sidney S, McCulloch CE, Young WL, Kim H. Angiopoietin-like 4 (ANGPTL4) gene polymorphisms and risk of brain arteriovenous malformations. Cerebrovasc Dis. 2011;31:338–345. doi: 10.1159/000322601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, Tan CK, Huang RL, Sze SK, Tang MB, Ding JL, Kersten S, Tan NS. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285:32999–33009. doi: 10.1074/jbc.M110.108175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci U S A. 2005;102:6086–6091. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 94.Ye J. Adipose tissue vascularization: its role in chronic inflammation. Curr Diab Rep. 2011;11:203–210. doi: 10.1007/s11892-011-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen W, Ma T, Shen XN, Xia XF, Xu GD, Bai XL, Liang TB. Macrophage-induced tumor angiogenesis is regulated by the TSC2-mTOR pathway. Cancer Res. 2012;72:1363–1372. doi: 10.1158/0008-5472.CAN-11-2684. [DOI] [PubMed] [Google Scholar]
- 97.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 98.Arras M, Ito WD, Scholz D, Winkler B, Schaper J, Schaper W. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J Clin Invest. 1998;101:40–50. doi: 10.1172/JCI119877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, Elner SG, Strieter RM. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992;258:1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 100.Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329:630–632. doi: 10.1038/329630a0. [DOI] [PubMed] [Google Scholar]
- 101.Stefater JA, 3rd, Rao S, Bezold K, Aplin AC, Nicosia RF, Pollard J, Ferrara N, Lang RA. Macrophage Wnt-Calcineurin-Flt1 signaling regulates mouse wound angiogenesis and repair. Blood. 2013 doi: 10.1182/blood-2012-06-434621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newman AC, Hughes CC. Macrophages and angiogenesis: a role for Wnt signaling. Vasc Cell. 2012;4:13. doi: 10.1186/2045-824X-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab. 2008;295:E313–322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Han J, Lee JE, Jin J, Lim JS, Oh N, Kim K, Chang SI, Shibuya M, Kim H, Koh GY. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 105.Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, Tallquist MD, Graff JM. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tang W, Zeve D, Seo J, Jo AY, Graff JM. Thiazolidinediones regulate adipose lineage dynamics. Cell Metab. 2011;14:116–122. doi: 10.1016/j.cmet.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tran KV, Gealekman O, Frontini A, Zingaretti MC, Morroni M, Giordano A, Smorlesi A, Perugini J, De Matteis R, Sbarbati A, Corvera S, Cinti S. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15:222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupta RK, Mepani RJ, Kleiner S, Lo JC, Khandekar MJ, Cohen P, Frontini A, Bhowmick DC, Ye L, Cinti S, Spiegelman BM. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15:230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wosnitza M, Hemmrich K, Groger A, Graber S, Pallua N. Plasticity of human adipose stem cells to perform adipogenic and endothelial differentiation. Differentiation. 2007;75:12–23. doi: 10.1111/j.1432-0436.2006.00110.x. [DOI] [PubMed] [Google Scholar]
- 110.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 111.Rajashekhar G, Traktuev DO, Roell WC, Johnstone BH, Merfeld-Clauss S, Van Natta B, Rosen ED, March KL, Clauss M. IFATS collection: Adipose stromal cell differentiation is reduced by endothelial cell contact and paracrine communication: role of canonical Wnt signaling. Stem cells. 2008;26:2674–2681. doi: 10.1634/stemcells.2008-0277. [DOI] [PubMed] [Google Scholar]
- 112.Ba K, Yang X, Wu L, Wei X, Fu N, Fu Y, Cai X, Yao Y, Ge Y, Lin Y. Jagged-1-mediated activation of notch signalling induces adipogenesis of adipose-derived stem cells. Cell Prolif. 2012;45:538–544. doi: 10.1111/j.1365-2184.2012.00850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fukuda D, Aikawa E, Swirski FK, Novobrantseva TI, Kotelianski V, Gorgun CZ, Chudnovskiy A, Yamazaki H, Croce K, Weissleder R, Aster JC, Hotamisligil GS, Yagita H, Aikawa M. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci U S A. 2012;109:E1868–1877. doi: 10.1073/pnas.1116889109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Garces C, Ruiz-Hidalgo MJ, Font de Mora J, Park C, Miele L, Goldstein J, Bonvini E, Porras A, Laborda J. Notch-1 controls the expression of fatty acid-activated transcription factors and is required for adipogenesis. J Biol Chem. 1997;272:29729–29734. doi: 10.1074/jbc.272.47.29729. [DOI] [PubMed] [Google Scholar]
- 115.Cantile M, Procino A, D’Armiento M, Cindolo L, Cillo C. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J Cell Physiol. 2003;194:225–236. doi: 10.1002/jcp.10210. [DOI] [PubMed] [Google Scholar]
- 116.Gesta S, Bluher M, Yamamoto Y, Norris AW, Berndt J, Kralisch S, Boucher J, Lewis C, Kahn CR. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103:6676–6681. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karastergiou K, Fried SK, Xie H, Lee MJ, Divoux A, Rosencrantz MA, Chang RJ, Smith SR. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab. 2013;98:362–371. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vohl MC, Sladek R, Robitaille J, Gurd S, Marceau P, Richard D, Hudson TJ, Tchernof A. A survey of genes differentially expressed in subcutaneous and visceral adipose tissue in men. Obes Res. 2004;12:1217–1222. doi: 10.1038/oby.2004.153. [DOI] [PubMed] [Google Scholar]
- 119.Stoll SJ, Kroll J. HOXC9: a key regulator of endothelial cell quiescence and vascular morphogenesis. Trends Cardiovasc Med. 2012;22:7–11. doi: 10.1016/j.tcm.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 120.Stoll SJ, Bartsch S, Augustin HG, Kroll J. The transcription factor HOXC9 regulates endothelial cell quiescence and vascular morphogenesis in zebrafish via inhibition of interleukin 8. Circ Res. 2011;108:1367–1377. doi: 10.1161/CIRCRESAHA.111.244095. [DOI] [PubMed] [Google Scholar]
- 121.Bruhl T, Urbich C, Aicher D, Acker-Palmer A, Zeiher AM, Dimmeler S. Homeobox A9 transcriptionally regulates the EphB4 receptor to modulate endothelial cell migration and tube formation. Circ Res. 2004;94:743–751. doi: 10.1161/01.RES.0000120861.27064.09. [DOI] [PubMed] [Google Scholar]
- 122.Bandyopadhyay S, Ashraf MZ, Daher P, Howe PH, DiCorleto PE. HOXA9 participates in the transcriptional activation of E-selectin in endothelial cells. Molecular and cellular biology. 2007;27:4207–4216. doi: 10.1128/MCB.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Trivedi CM, Patel RC, Patel CV. Homeobox gene HOXA9 inhibits nuclear factor-kappa B dependent activation of endothelium. Atherosclerosis. 2007;195:e50–60. doi: 10.1016/j.atherosclerosis.2007.04.055. [DOI] [PubMed] [Google Scholar]
- 124.Rhoads K, Arderiu G, Charboneau A, Hansen SL, Hoffman W, Boudreau N. A role for Hox A5 in regulating angiogenesis and vascular patterning. Lymphat Res Biol. 2005;3:240–252. doi: 10.1089/lrb.2005.3.240. [DOI] [PubMed] [Google Scholar]
- 125.Zhu Y, Cuevas IC, Gabriel RA, Su H, Nishimura S, Gao P, Fields A, Hao Q, Young WL, Yang GY, Boudreau NJ. Restoring transcription factor HoxA5 expression inhibits the growth of experimental hemangiomas in the brain. J Neuropathol Exp Neurol. 2009;68:626–632. doi: 10.1097/NEN.0b013e3181a491ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamamoto Y, Gesta S, Lee KY, Tran TT, Saadatirad P, Kahn CR. Adipose depots possess unique developmental gene signatures. Obesity (Silver Spring) 2010;18:872–878. doi: 10.1038/oby.2009.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hodson L, Humphreys SM, Karpe F, Frayn KN. Metabolic Signatures of Human Adipose Tissue Hypoxia in Obesity. Diabetes. 2012 doi: 10.2337/db12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rupnick MA, Panigrahy D, Zhang CY, Dallabrida SM, Lowell BB, Langer R, Folkman MJ. Adipose tissue mass can be regulated through the vasculature. Proc Natl Acad Sci U S A. 2002;99:10730–10735. doi: 10.1073/pnas.162349799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Daquinag AC, Zhang Y, Kolonin MG. Vascular targeting of adipose tissue as an anti-obesity approach. Trends Pharmacol Sci. 2011;32:300–307. doi: 10.1016/j.tips.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 130.Alligier M, Gabert L, Meugnier E, Lambert-Porcheron S, Chanseaume E, Pilleul F, Debard C, Sauvinet V, Morio B, Vidal-Puig A, Vidal H, Laville M. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. J Clin Endocrinol Metab. 2013;98:802–810. doi: 10.1210/jc.2012-3289. [DOI] [PubMed] [Google Scholar]
- 131.Spencer M, Unal R, Zhu B, Rasouli N, McGehee RE, Jr, Peterson CA, Kern PA. Adipose tissue extracellular matrix and vascular abnormalities in obesity and insulin resistance. J Clin Endocrinol Metab. 2011;96:E1990–1998. doi: 10.1210/jc.2011-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tan CY, Vidal-Puig A. Adipose tissue expandability: the metabolic problems of obesity may arise from the inability to become more obese. Biochemical Society transactions. 2008;36:935–940. doi: 10.1042/BST0360935. [DOI] [PubMed] [Google Scholar]
- 133.Strickland LR, Guo F, Lok K, Garvey WT. Type 2 Diabetes With Partial Lipodystrophy of the Limbs: A new lipodystrophy phenotype. Diabetes Care. 2013 doi: 10.2337/dc12-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Haque WA, Oral EA, Dietz K, Bowcock AM, Agarwal AK, Garg A. Risk factors for diabetes in familial partial lipodystrophy Dunnigan variety. Diabetes Care. 2003;26:1350–1355. doi: 10.2337/diacare.26.5.1350. [DOI] [PubMed] [Google Scholar]
- 135.Joffe BI, Panz VR, Raal FJ. From lipodystrophy syndromes to diabetes mellitus. Lancet. 2001;357:1379–1381. doi: 10.1016/S0140-6736(00)04616-X. [DOI] [PubMed] [Google Scholar]
- 136.Khan T, Muise ES, Iyengar P, Wang ZV, Chandalia M, Abate N, Zhang BB, Bonaldo P, Chua S, Scherer PE. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Molecular and cellular biology. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. The Journal of clinical investigation. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Beranger GE, Karbiener M, Barquissau V, Pisani DF, Scheideler M, Langin D, Amri EZ. In vitro brown and “brite”/“beige” adipogenesis: Human cellular models and molecular aspects. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbalip.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 139.Bonet ML, Oliver P, Palou A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta. 2012 doi: 10.1016/j.bbalip.2012.12.002. [DOI] [PubMed] [Google Scholar]


