Figure 8.
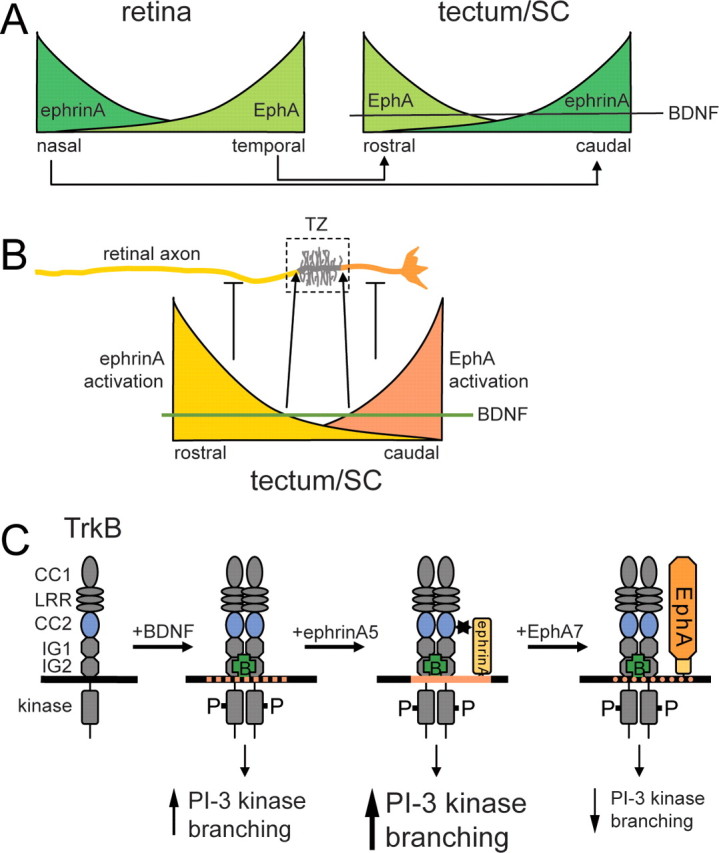
Model for the topographic branching of retinal axons in the tectum/SC based on an interaction between ephrinAs and TrkB. A, Schematic expression pattern of EphAs and ephrinAs in the retina (left) and tectum/SC (right). BDNF is uniformly expressed in the tectum/SC and promotes branching of retinal axons globally via TrkB. The axonal projection pattern is indicated with nasal axons projecting onto the posterior tectum/SC and temporal axons onto the anterior tectum/SC. B, EphAs and ephrinAs, as well as TrkB receptors, are uniformly distributed over the entire length of the axon. Local activation of ephrinAs on retinal axons in the proximal part of the axon and of EphAs in the distal part of the axon, causing a corresponding local suppression of branching resulting in the formation of a termination zone in that part of the axon in which branch suppression is minimal. Suppression anterior to the termination zone: ephrinAs are activated locally by tectally expressed EphAs. Activated ephrinAs in turn abolish BDNF-mediated branching. Because of the anterior > posterior gradient of EphAs, branch suppression occurs anterior (proximal) to the termination zone. Suppression posterior to the termination zone: it appears plausible that this activity is controlled by gradients of ephrinAs in the posterior tectum/SC (Yates et al., 2001), which activate retinal EphA receptors and which might interfere with TrkB signaling (Kong et al., 2001; Fitzgerald et al., 2008). C, Effects of ephrin/TrkB interactions on retinal axon branching. Binding of BDNF to TrkB results in its activation and tyrosine phosphorylation and leads via activation of, in particular, PI-3 kinase signaling to an increase in branching. Interaction of activated TrkB with ephrinAs (possibly in lipid rafts; orange) augments PI-3 kinase signaling and branching. Activation of ephrinAs by EphAs diminishes branching and a reduction in pAkt signaling.
