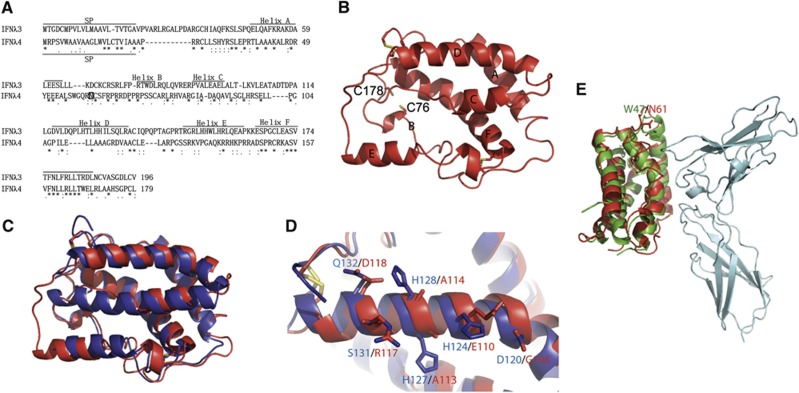
Figure 3.
Homology model of human interferon lambda 4. (A) Alignment of IFNλ3 and IFNλ4 using Clustal W. The position of the signal peptide (SP) in IFNλ3 and IFNλ4 is shown. The positions of the helices in IFNλ3 and in the model of IFNλ4 shown in (B) are shown. A possible N-linked glycosylation site at Asn61 is marked by a square in IFNλ4. (B) Homology model of IFNλ4 (homIFNλ4) generated by the Swiss model server using IFNλ3 (PDB entry code HHC3) as a template. The position of the individual structural elements is denoted as A–F. The cysteins are shown as yellow sticks. The positions of C76 and C178 that could form a disulphide bridge specific for IFNλ4 are shown. (C) Superimposition of IFNλ3 (blue) and homIFNλ4 (red). (D) Comparison of helix D of IFNλ3 (blue) and homIFNλ4 (red). The residues that are expected to interact with IL-10R2 are labelled in blue for IFNλ3 and red for IFNλ4. Disulphides are shown in yellow. (E) Superimposition of homIFNλ4 (red) onto IFNλ1 (green) bound to IFNλR1 (cyan). N61 of homIFNλ4 (red) and W47 of IFNλ1 (green) are shown in sticks and labelled.