Figure 2.
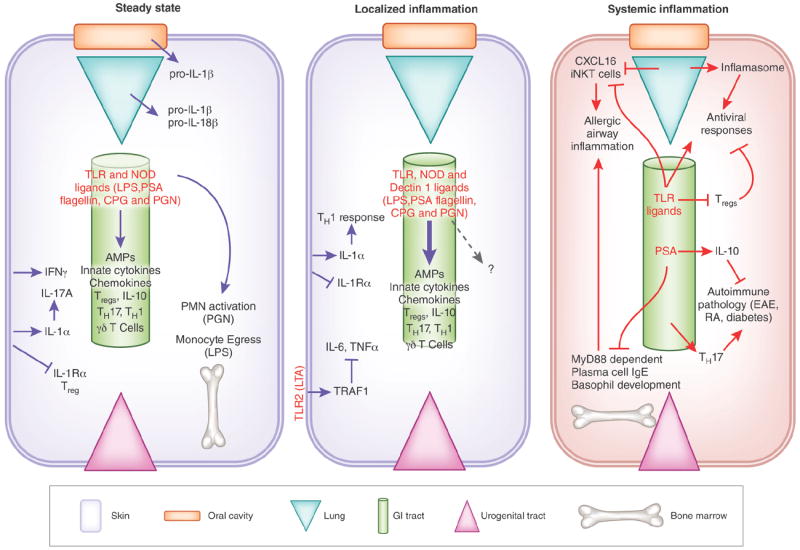
Localized and systemic regulation of the immune system by distinct commensal niches. (a) Commensal bacteria in the skin dynamically regulate the cutaneous effector and Treg cells by amplifying inflammatory signals (IL-1). Similarly, commensal signals in the oral mucosa and respiratory tract promote the production of IL-1, and of IL-1 and IL-18, respectively. In the gastrointestinal tract, commensal ligands (TLR, NLR and Nod ligands) and commensal metabolites (short-chain fatty acids) instruct immunity locally. Trace amounts of commensal byproducts enter blood circulation and localize to the bone marrow where they control immune cell development and function. (b) Skin commensals control protective TH1 responses during a dermal infection in an IL-1–dependent manner. A TLR2 ligand, lipoteichoic acid (LTA) specifically derived from S. epidermidis ameliorates exuberant production of TNF and IL-6 during skin inflammation. The signals involved in regulation of intestinal immunity during localized inflammatory responses are similar to those involved in controlling immune homeostasis, but these commensal derived signals are greatly amplified during an inflammatory response. Signals from gut microbiota may diffuse more readily into systemic circulation during gut inflammation. (c) Intestinal microbiota has been identified as a key modulator of systemic immunity. Gut-dwelling commensals can promote pathology in various mouse models of autoimmunity (experimental autoimmune encephalomyelitis (EAE), diabetes and arthritis). The intestinal microbiota also regulates viral immunity in the lung by controlling macrophage responses and inflammasome activation. Allergic airway inflammation is negatively regulated by signals from the flora that down-modulate responses of immune effectors, including production of IgE by plasma cells, development of basophils and accumulation of iNKT in the gut and lung. innate Natural Killer T cells (iNKT), experimental autoimmune encephalomyelitis (EAE), Rheumatoid arthritis (RA), polysaccharide A(PSA), polymorphonuclear neutrophils (PMN), Peptidoglycan (PGN), Antimicrobial peptides (AMPs).
