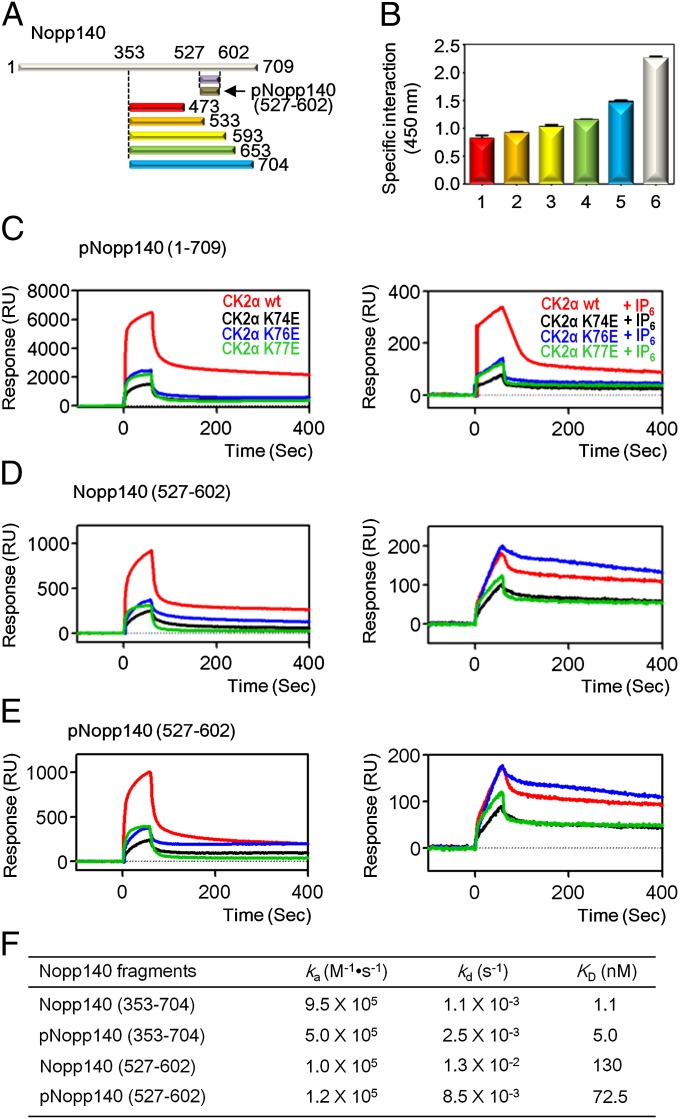
Fig. 1.
Mapping binding region of Nopp140 with CK2α and the inhibitory effect of IP6. (A) Schematic representation of Nopp140 constructs. (B) Specific interaction between C-terminal domain of Nopp140 and CK2α observed using a modified ELISA method. Purified CK2α was immobilized on a plate, and the intensities of the chemiluminescence signal from the bound HRP-conjugated anti–His-tag antibody with His-Nopp140 fragments, 353–473 (red), 353–533 (orange), 353–593 (yellow), 353–653 (green), 353–704 (cyan), and 1–709 (white) were measured. (C) SPR sensorgrams of pNopp140 (1–709) with CK2α, CK2α wild type (colored in red), and CK2α mutants (K74E in black, K76E in blue, and K77E in green) were used as analytes with (Right) and without IP6 (Left). (D) SPR sensorgrams of Nopp140 (527–602) with CK2α. The analytes were used as indicated in Fig. 1C. (E) SPR sensorgrams of pNopp140 (527–602) with CK2α. The analytes were used as indicated in Fig. 1C. (F) Kinetic data for the interaction between CK2α wild-type and Nopp140 fragments. Original SPR sensorgrams are shown in Fig. S3.