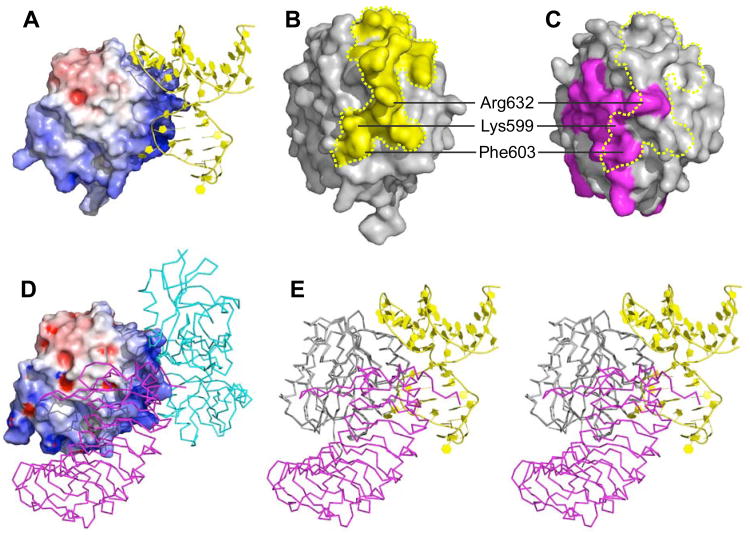
Figure 5. Structural basis for the antithrombotic activity of ARC1172.
(A) ARC1172-A1 complex (PDB code: 3XHO) with the surface of A1 domain colored according to negative (red) and positive (blue) electrostatic potential. (B) The surface of VWF A1 (Phe507-Thr705) within 4.2 Å of ARC1172 is colored yellow.(C) The surface of VWF A1 (PDB code: 1M10) in contact with GPIα is colored magenta. The yellow dashed line encompasses the surface that is in contact with ARC1172 as in panel B. (D) Surface of VWF A1 domain (Asp498-Thr705) in complex with botrocetin (cyan) and GPIbα (magenta) (PDB ID: 1U0N) showing that botrocetin and ARC1172 occupy similar sites. The surfaces of A1 domain in (A) and (D) are slightly different due to small differences in side chain positions and also to the different length and orientation of the N-terminal residues at the bottom of the structures. (E) Stereo view of VWF A1 complexed with ARC1172 (yellow cartoon) and GPIbα (magenta, PDB ID: 1M10) would clash upon binding to VWF A1 domain. The clashing residues are listed in Table S3. The A1 domain has the same orientation in panels A, D, and E, and is turned approximately 90° around the vertical axis in panels B and C.