Figure 1. The ApaG Fbxo3 domain serves as a target for small molecules.
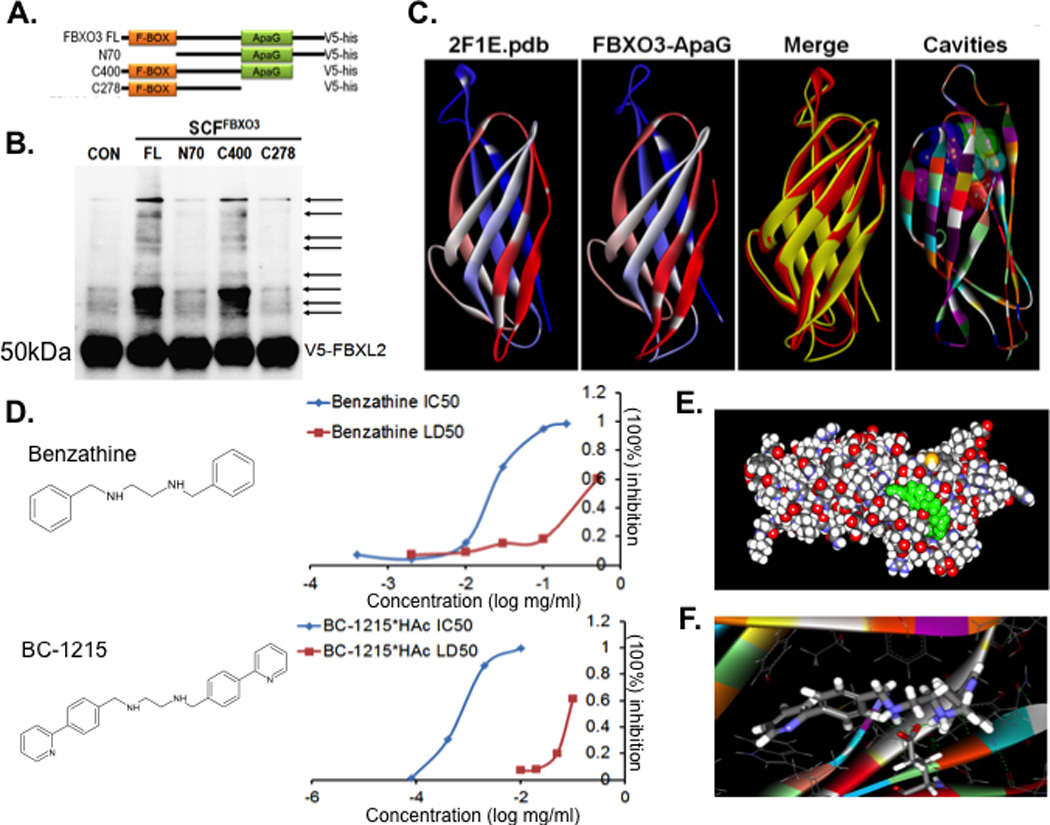
A. Several deletion mutants of Fbxo3 were designed and cloned into a pcDNA3.1D/V5-HIS vector. B. In vitro ubiquitination assays. Purified SCFFbxo3 full-length (FL) or truncated Fbxo3 proteins were incubated with V5-Fbxl2 substrate and the full complement of ubiquitination reaction components showing polyubiquitinated Fbxl2 (second lane from left). C. Structural analysis of the Fbxo3-ApaG domain showing highly conserved bacterial (left) and mammalian Fbxo3 (second from left) structures. D. Structure of the base compound, benzathine, and the Fbxo3 antagonist, BC-1215. Right graphs. Human peripheral blood mononuclear (PBMC) cells were treated with LPS (2 µg/ml) for 16 h along with either benzathine or BC-1215 at different concentrations and IL1β and TNFα monitored to calculate the IC50. For LD50, U937 monocytes were treated with the small molecules at different concentrations for 16 h. Cells were then stained with trypan blue to identify dead cells, and to calculate the LD50. E–F. Docking studies of the base compound, benzathine, interacting with the Fbxo3-ApaG domain. Data from panel B represent n=2 separate experiments.
