Figure 2. A small molecule inhibitor, BC-1215, inhibits Fbxo3 function.
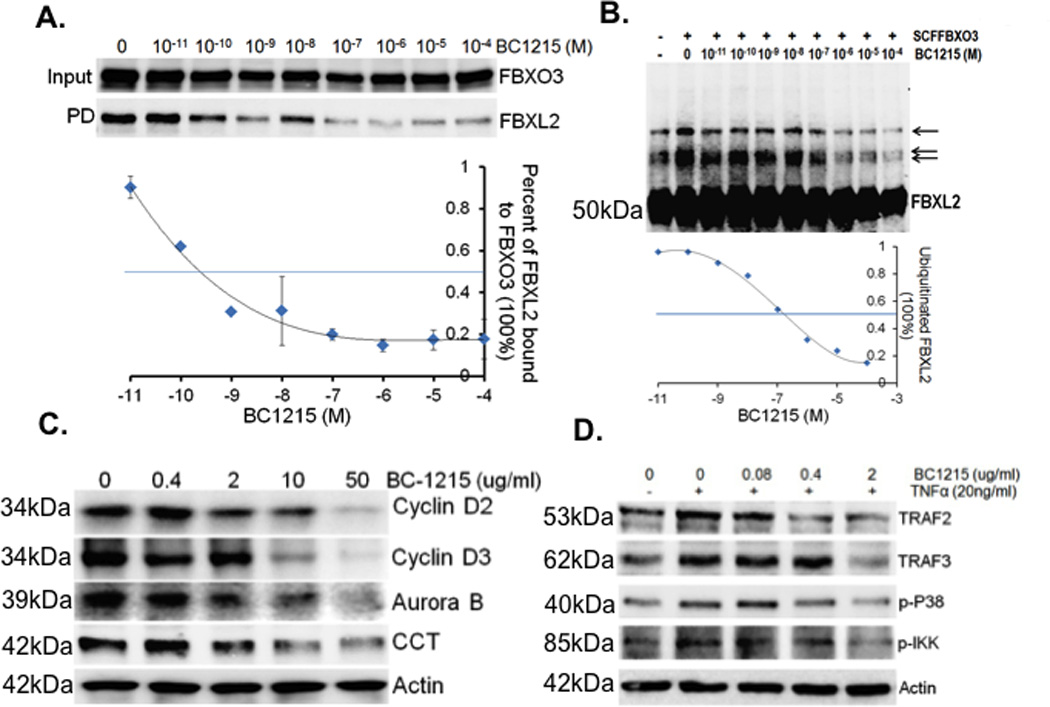
A. Fbxo3 protein was immunoprecipitated using Fbxo3 antibody and captured with protein A/G beads from Hela cell lysates. Fbxo3 beads were then extensively washed prior to exposure to BC-1215 at different concentrations (10−11 to 10−4 M). Purified FBXL2 protein was then incubated with Fbxo3 beads overnight, beads were washed, and F box complexes were eluted and resolved on SDS-PAGE. The relative amounts of Fbxl2 detected in pull-downs (PD) was normalized to loading and quantified as shown graphically below. B. In vitro ubiquitination assays. Purified SCFFbxo3 complex components were incubated with V5-Fbxl2 and the full complement of ubiquitination reaction components with increased concentrations of BC-1215 or vehicle showing decreased levels of polyubiquitinated Fbxl2 (arrows). The lower graph quantifies levels of ubiquitinated Fbxl2 as a function of BC-1215 concentration. C. Murine lung epithelial (MLE) cells were treated with BC-1215 at different concentrations for 16 h. Cells were collected and assayed for cyclin D2, cyclin D3, Aurora B, CCT, and β actin immunoblotting. D. MLE cells were co-treated with BC-1215 (10 µg/ml) and TNFα (10 ng/ml) for 6 h. Cells were then collected and assayed for immunoblotting. Data from each panel represents at least n=2 separate experiments.
