Abstract
The network for cardiac fuel metabolism contains intricate sets of interacting pathways that result in both ATP producing and non-ATP producing end-points for each class of energy substrates. The most salient feature of the network is the metabolic flexibility demonstrated in response to various stimuli, including developmental changes and nutritional status. The heart is also capable of remodeling the metabolic pathways in chronic pathophysiological conditions, which results in modulations of myocardial energetics and contractile function. In a quest to understand the complexity of the cardiac metabolic network, pharmacological and genetic tools have been engaged to manipulate cardiac metabolism in a variety of research models. In concert, a host of therapeutic interventions have been tested clinically to target substrate preference, insulin sensitivity, and mitochondrial function. In addition, the contribution of cellular metabolism to growth, survival, and other signaling pathways through the production of metabolic intermediates has been increasingly noted. In this review, we provide an overview of the cardiac metabolic network and highlight alterations observed in cardiac pathologies as well as strategies employed as metabolic therapies in heart failure. Lastly, the ability of metabolic derivatives to intersect growth and survival are also discussed.
Keywords: Cardiac metabolism, cardiac pathology, metabolic signaling, metabolic therapy
Introduction
The mammalian heart must contract incessantly, thus, the requirement for energy to fuel optimal function is immense. As the high energy phosphate storage within the cardiomyocyte is minimal, only sufficient to sustain the heart beat for a few seconds, a tight coupling of ATP production and myocardial contraction is essential for normal cardiac function. Central to the coordinated energy transduction function is the multi-purpose organelle mitochondrion which not only generates more than 95% of ATP utilized by the heart but also regulates intracellular calcium homeostasis, signaling and cell death. While a constant supply of substrates through the metabolic network is paramount for mitochondrial conversion of ATP, it is increasingly recognized that metabolites generated by both ATP-producing and non-ATP producing pathways can become critical regulators of cell function. Thus, the importance of substrate metabolism to cardiac pump function is beyond the scope of only modulation of energy supply. In this article, we will provide an overview of changes in cardiac fuel metabolism under pathological conditions followed by recent progress on targeting cardiac metabolism for improving myocardial energetics and function. In addition, we will summarize the emerging role of cardiac metabolism in governing myocardial growth and survival pathways.
Characteristics of Fuel Metabolism in the Heart
The capacity and flexibility of substrate metabolism for ATP production
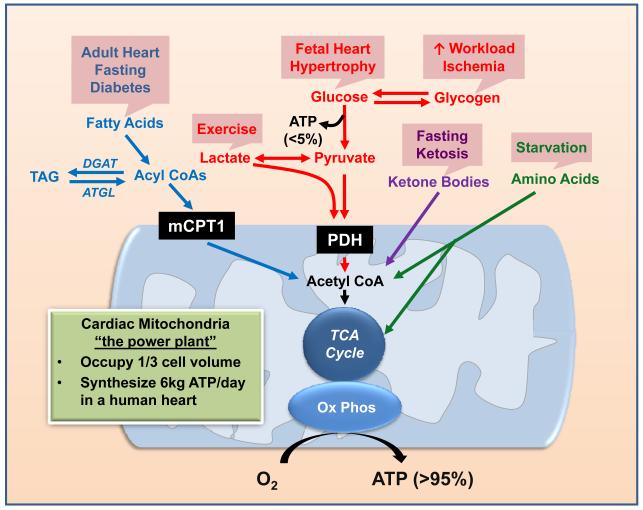
The heart is capable of utilizing all classes of energy substrates, including carbohydrates, lipids, amino acids and ketone bodies, for ATP production in the mitochondrion (Figure 1, for details see reviews1-3). Mitochondria occupy one third of the cell volume in cardiac myocytes making them the cell type with the highest mitochondria content.4 The robustness of cardiac metabolism is reflected by its highest oxygen consumption rate on the per unit weight basis. For a human heart, the amount of ATP turned over during a one-day period is 15-20 times of its own weight. In a normal heart, mitochondria are largely fueled by fatty acyl-CoA and pyruvate, which are the primary metabolites of fatty acids and carbohydrates, respectively. The entry of long-chain acyl-CoA into the mitochondrion is a regulated process; with the rate-limiting step at the muscle form of the carnitine-palmitoyl transferase I (mCPT-1) reaction. The oxidation of pyruvate is regulated at the pyruvate dehydrogenase (PDH) reaction. Other substrates, including lactate, ketone bodies and amino acids, can enter mitochondria directly for oxidation. Metabolism of ketone bodies yields acetyl-CoA while amino acid catabolism yields keto-acids which are further metabolized to enter the TCA cycle. The contribution of ketone bodies and amino acids to overall cardiac oxidative metabolism is considered to be minor due to the low availability of these substrates under normal physiological conditions.5-7
Figure 1. Overview of the Metabolic Network.
The energy-yielding substrates (fatty acids, glucose, ketones, and amino acids), via specific catabolic pathways, converge on acetyl CoA production with subsequent entry into the tricarboxylic acid (TCA) cycle. The final step of energy transfer is accomplished through oxidative phosphorylation (OxPhos), supplying greater than 95% of ATP consumed by the heart. The boxes (in pink) above each metabolic pathway indicate the pathological and/or physiological condition in which the specific substrate becomes a predominant contributor to metabolism. TAG, triacylglycerol; DGAT, diacylglycerol acyltransferase; ATGL, adipose triglyceride lipase; mCPT1, muscle form of carnitine palmitoyl transferase; PDH, pyruvate dehydrogenase; TCA, tricarboxcylic acid; O2, oxygen.
It is widely accepted that fatty acids are the predominant substrate utilized in the adult myocardium. However, the cardiac metabolic network is highly flexible in utilizing other substrates when they become abundantly available (Figure 1). For example, cardiac extraction and oxidation of lactate becomes predominant during exercise as skeletal muscle lactate production increases.8,9 Prolonged fasting or ketogenic diet increases the blood level of ketone bodies and results in enhanced utilization by the heart.7 In isolated perfused hearts, the addition of lactate or ketone bodies in the perfusate significantly reduces the oxidation of glucose and fatty acids.5,10,11 These studies support the concept of metabolic flexibility that confers the advantage of adequately supplying ATP for continual cardiac contraction under a variety of physiological conditions.
Apart from substrate availability, complex regulatory mechanisms contribute to metabolic flexibility at multiple levels, including transcriptional regulation and post-translational modification of key proteins involved in each metabolic pathway as well as allosteric regulation by substrates and their metabolites (Table 1). For example, transcriptional regulation of the proteins involved in fatty acid oxidation (FAO) by the PPAR/ERR/PGC-1 circuit is a major mechanism in the transition of the glycolysis-dependent fetal heart to oxidative metabolism in the adult heart.12-14 Likewise, transcriptional regulation by HIF1α is responsible for the metabolic adaptation to hypoxic and ischemic conditions.15 While transcriptional mechanisms contribute to the establishment of the network, post-translational modifications of key enzymes in the metabolic pathways regulate the fluxes. Phosphorylation and inactivation of PDH by pyruvate dehydrogenase kinase 4 (PDK4) plays a key role in the shift of substrate oxidation between glucose and fatty acid in the heart.16 The phosphorylation of the branched-chain-α-ketoacid dehydrogenase (BCKD), regulated by BCKD kinase and a mitochondrial localized phosphatase (PP2Cm), governs the oxidation of branch amino acids.17,18
Table 1. Regulators of Substrate Metabolism.
Known factors of transcription, protein modification, and allosteric regulators involved in the stimulation or inhibition of metabolic pathways. Numbers indicate relevant references for review. HIF1α, hypoxia-inducible factor 1-alpha, PPARγ, peroxisome proliferator-activated receptor gamma; AMPK, AMP-activated protein kinase; F1,6BP, fructose 1,6-biphosphate; Pi, inorganic phosphate; NAD+, nicotinamide adenine dinucleotide; NADH, nicotinamide adenine dinucleotide, reduced; G6P, glucose 6-phosphate; FOXO1, forkhead box protein O1; PDK4, pyruvate dehydrogenase kinase 4; PCG-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; ERRα, estrogen-related receptor alpha; MCD, malonyl CoA decarboxylase; ACC2, acetyl CoA carboxylase 2; PP2Cm, protein phosphatase 2Cm; BCKDK, branched chain ketoacid dehydrogenase kinase.
| Pathway | Stimulation | Inhibition |
|---|---|---|
| Glycolysis | HIF1α15 PPARγ209 AMPK210 Insulin211 Epinephrine212 AMP, ADP, Pi213 NAD+ 213 F1,6BP213 |
ATP213 NADH213 G6P213 citrate214 |
| Glucose oxidation | Insulin215 Epinephrine212 NAD+213 Calcium216 |
PPARα209 FOXO1217 PDK416 Fatty Acids214 Acetyl CoA, NADH, ATP213 |
|
| ||
| Fatty acid oxidation | PPAR/PGC-1α/ERR12-14,209 FOXO1217 AMPK210 MCD218 Adiponectin219,220 Fatty Acids221 |
ACC2222 Malonyl-CoA223 Glucose214 Lactate11 Ketone bodies10 |
|
| ||
| BCAA catabolism | PP2Cm17,18 Glucagon224 |
BCKDK225 NADH, CoA esters213 |
| Ketone body oxidation |
acetoacetate213 | |
Intermediates of glucose metabolism
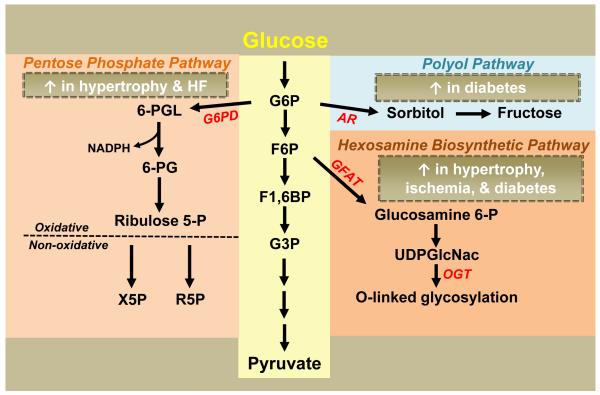
Although glucose catabolism through glycolysis primarily yields pyruvate for subsequent oxidation, glycolytic intermediates can participate in several additional pathways that do not lend to ATP generation (Figure 2). These pathways are of biological significance in the heart despite the small fluxes. Glucose 6-phosphate (G6P) produced by the hexokinase reaction enters the Pentose Phosphate Pathway (PPP), yielding NADPH during the oxidative phase and 5-carbon sugars in the subsequent non-oxidative phase.19 The supply of NADPH from the PPP is important for antioxidant defense as NADPH is required for maintaining the level of reduced glutathione.20 It has been shown that deficiency of G6P dehydrogenase (G6PD), the first and rate-limiting enzyme of the PPP, exacerbates ischemia-reperfusion injury in mice,21 indicating a protective role of the PPP against oxidative injury. End products of the non-oxidative phase of the PPP are also of significance as ribose 5-phosphate becomes a substrate for nucleotide or nucleic acid synthesis22 while xylulose 5-phosphate has been suggested as a transcriptional signaling molecule.23,24
Figure 2. Accessory Pathways of Glucose Metabolism.
Multiple accessary pathways of glucose metabolism result in the production of metabolites that do not directly contribute to energy supply but are of important biological function. Evidence has suggested that these pathways are altered in the hypertrophied, failing, ischemic, and/or diabetic heart as indicated. Glycolysis: G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; F1,6BP, fructose 1,6-biphosphate; G3P, glyceraldehyde 3-phosphate. Pentose Phosphate Pathway (PPP): G6PD, glucose 6-phosphate dehydrogenase; 6-PGL, 6-phosphoglucono-δ-lactone; NADPH, nicotinamide adenine dinucleotide phosphate; 6-PG, 6-phosphogluconate; Ribulose 5-P, ribulose 5-phosphate; X5P, xylulose 5-phosphate, R5P, ribose 5-phosphate. Polyol Pathway: AR, aldose reductase. Hexosamine Biosynthetic Pathway (HBP): GFAT, glutamine fructose 6-phosphate amidotransferase; Glucosamine 6-P, glucosamine 6-phosphate; UDPGlcNAc, uridine diphosphate-N-acetylglucosamine; OGT, O-linked β-N-acetylglucosamine transferase.
An alternative fate of G6P is the production of sorbitol, via the enzyme aldose reductase (AR), in the polyol pathway. The role of the polyol pathway in normal cardiac metabolism is unknown. However, increased flux has been noted in diabetic patients and has been associated with abnormal glucose metabolism and cardiac dysfunction.25,26 Increased AR flux has also been implicated in the myocardial response to ischemia-reperfusion injury.27,28 Elucidation of the role of the polyol pathway in mouse models should be used with caution as both expression and activity of AR are significantly lower in mice than in humans; however, the use of transgenic mice overexpressing human AR could be translatable.28
The glycolytic intermediate fructose 6-phosphate (F6P) can diverge into the hexosamine biosynthetic pathway (HBP), yielding uridine diphosphate-N-acetylglucosamine (UDPGlcNAc), via the enzyme glutamine fructose 6-phosphate amidotransferase (GFAT).29 UDPGlcNAc is used as the substrate for O-linked-GlcNAc transferase (OGT) which catalyzes the OGlcNAcylation of proteins.30 Increases in protein O-linked GlcNAcation have been observed in diabetes and proposed to be responsible for altered insulin sensitivity and FAO.30,31 Recent studies show that protein O-linked GlcNAcation is enhanced during ischemia-reperfusion and represents a cardioprotective mechanism against injury.32-34
The turnover of endogenous substrates
The heart stores fuel in the form of glycogen and triacylglycerol (TAG, Figure 1). The turnover rate of the cardiac glycogen pool is rather low under normal conditions in the adult heart.35,36 Glycogen metabolism has an essential role in the fetal heart as the absence of glycogen due to the deletion of glycogen synthase (GYS), causes abnormal cardiac development.37 Glucose derived from glycogenolysis also provides a critical energy supply for cell survival during ischemia.38 Glycogen is also a key energy source to support metabolism during acute increases in cardiac workload.35
The turnover of cardiac TAG is more robust compared to the glycogen pool although its functional role has been less understood until recently.39,40 It has been postulated that fatty acids derived from the myocardial TAG pool can be oxidized and contribute ~10% to the total ATP production under normal physiological conditions.41,42 A significant loss of TAG turnover was observed in hearts from failing rats41 while accelerated turnover was noted in diabetic rats.43 These results suggest that the intracellular TAG pool is a dynamic entity but the functional significance of altered TAG turnover under pathological conditions is poorly understood.
Recent studies suggest that the turnover of TAG pool is an important regulatory mechanism of fatty acid metabolism in the myocardium. Genetic manipulation of diacylglycerol acyltransferase (DGAT), the final enzyme in synthesis of TAG from diacylglycerol (DAG), or adipose triglyceride lipase (ATGL), the enzyme responsible for TAG hydrolysis, leads to significant changes of fatty acid uptake and oxidation in the mouse heart. Although deletion of DGAT1, the major isoform of DGAT in the heart, did not significantly decrease total cardiac TAG content, it was associated with decreased FAO and increased glucose uptake.44 Conversely, DGAT1 overexpression resulted in a two-fold increase in cardiac TAG with both increased uptake and oxidation of exogenous fatty acids.45 Global deletion of ATGL resulted in massive lipid accumulation and severe cardiomyopathy associated with decreased FAO.46,47 However, cardiac-specific overexpression of ATGL also led to decreased rates of FAO with increased rates of glucose oxidation suggesting a non-linear relationship between TAG turnover and FAO.48
Modulation of Contractile Function by Cardiac Metabolism Under Pathological Conditions
Pathological hypertrophy and failure
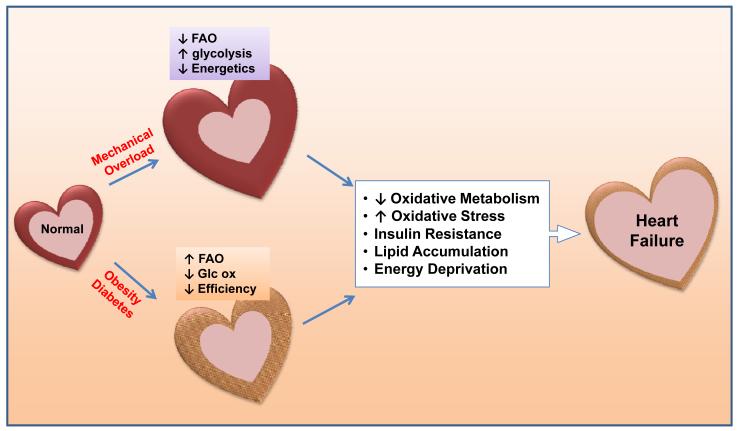
It is well established that cardiac metabolism undergoes a reprogramming in response to pathological hypertrophy, characterized by increased reliance on glucose metabolism and decreased FAO (Figures 2 and 3).49-52 Increased glucose utilization in the hypertrophied heart is predominantly characterized as an upregulation of glucose uptake and glycolysis50,53 with either no change or a decrease in glucose oxidation.36,54-56 These changes, combined with decreases in overall FAO, likely represent reduced capacity for mitochondrial oxidative metabolism. In small animal models, the shift in substrate preference is associated with downregulation of the transcriptional mechanisms for FAO and mitochondrial biogenesis mediated via PPARα and PGC-1.57 Because these changes resemble a reversal of metabolic maturation during the transition from a fetal to adult heart, many have considered the metabolic changes in hypertrophied and failing hearts as a reappearance of the fetal metabolic profile. Is there any advantage of switching to a fetal-like metabolism in the hypertrophied and failing myocardium? A shift from FAO to glucose utilization improves oxygen efficiency for ATP synthesis and is thus considered beneficial.58,59 This becomes particularly important for heart failure caused by chronic ischemic cardiomyopathy where oxygen supply is limited. There have been concerns whether increased glucose uptake and utilization in adult heart impairs cardiac function since cardiomyocytes cultured in high glucose media develop “glucotoxicity”.60-63 Transgenic mice with cardiac-specific overexpression of insulin-independent glucose transporter (GLUT1) showed substantial increases of glucose uptake and glycolysis but maintained normal cardiac function and lifespan suggesting that increased glucose utilization does not harm the adult heart in the longterm.64 A key question remaining is whether metabolic remodeling is adaptive or maladaptive to the high energy demand in the hypertrophied and failing heart. It is known that pathological cardiac hypertrophy is associated with depletion of energy reserves manifested as maintained ATP levels but a reduction of the energy reserve compound, phosphocreatine (PCr).65,66 This is reflected as a decreased PCr/ATP ratio and eventually, as compensated hypertrophy advances to overt heart failure, significant decreases of ATP are observed.67,68 The PCr/ATP ratio has been shown to be a superior predictor of mortality as compared to ejection fraction in heart failure patients 69 which is in agreement with the longstanding hypothesis that energy starvation contributes to the pathogenesis and progression of heart failure (See reviews67,68,70). These observations suggest that the fetal-like metabolic profile in cardiac hypertrophy is maladaptive for sustaining myocardial energetics and function (Figure 3).
Figure 3. Metabolic Remodeling and the Development of Heart Failure.
Pathological hypertrophy in response to mechanical overload, e.g. hypertension, valvular disease or post-MI, is accompanied by metabolic remodeling characterized by decreases in fatty acid oxidation (FAO) and increases in glycolysis. This fetal-like metabolic profile decreases the capacity for ATP synthesis, consistent with the “energy starvation” model. In contrast, the elevated supply of substrates in the heart of obese and/or diabetic individuals leads to an upregulation of FAO with a concomitant decrease in glucose oxidation (Glc ox). This lipid overload condition impairs cardiac efficiency. Regardless of the precipitating factor, the persistent metabolic derangements elicit commonalities of decreased oxidative metabolism, increased oxidative stress, insulin resistance, lipid accumulation, and energy deprivation, all contributing to the progression of heart failure.
Animal studies have shown that metabolic remodeling in hypertrophied heart is associated with decreases in the overall ATP synthesis by oxidative metabolism.50 Although glycolysis is increased, its contribution to total ATP synthesis is limited as glycolytic ATP accounts for less than 5% of total energy used by the heart.50 Furthermore, glucose entry in the adult heart is controlled by insulin; the co-existing insulin resistance in heart failure would limit the glucose availability and hence compromise the capacity for ATP synthesis.71,72 A proof of concept study shows that increasing glucose uptake capacity in mouse heart via an insulin-independent mechanism delays the transition of cardiac hypertrophy to failure.73 Other studies show that cardiac energetics and function can also be preserved in rodent models of heart failure by sustaining FAO or by enhancing ATP synthesis and transfer via the creatine kinase reaction.74-76 Therefore, the ATP synthesis capacity appears to be more important than the substrate selection for sustaining cardiac energetics and function in these models.
In addition to glycolysis and pyruvate oxidation, multiple accessory pathways of glucose metabolism (Figure 2) have also been altered in the hypertrophied myocardium. Increased flux of the anaplerotic pathway, primarily via increased malic enzyme, have been reported in hypertrophied rodent heart.76-78 Such a change is considered maladaptive as it “short-circuits” pyruvate into the second half of the TCA cycle and hence produces less NADH for oxidative phosphorylation. The regulatory enzyme of the PPP, G6PD, was upregulated in the hearts of animals subjected to pressure-overload.19,74 Increased activity of G6PD in heart failure was linked to excessive NAPDH and increased superoxide production.79,80 On the other hand, G6PD deficiency deprived NADPH supply for glutathione reduction leading to increased redox stress and exacerbated LV dilation and cardiac dysfunction in mice.81 It is likely that either excessive or deficient production of NADPH through the G6PD reaction impairs the redox regulation. It is also proposed that increased glucose utilization will augment HBP flux resulting in enhanced O-GlcNAcation.82 Increased glucose metabolism also elevates the citrate level in the cytosol providing more acetyl-CoA for the acetylation of proteins.83,84 It remains to be determined whether these changes contribute to the maladaptive nature of increased glucose utilization in heart failure. These pathways have been less investigated because their fluxes are small and they do not contribute to the once ultimate goal of cardiac metabolism, ATP production. However, given the emerging significance of non-ATP producing pathways in cardiac biology, this paradigm is rapidly changing. We shall expect a wealth of information in this regard in the future.
Metabolic cardiomyopathy associated with obesity and diabetes
In obese or diabetic individuals, cardiac dysfunction observed independent of macro- and/or microvascular disease is considered a consequence of “diabetic cardiomyopathy”. Increased fatty acid uptake and oxidation associated with reduced glucose oxidation have been observed in both animal models and patients of type 2 diabetes.85-87 Cardiac dysfunction in obesity and diabetes has been associated with increased myocardial oxygen consumption (MVO2), reduced cardiac efficiency, and increased oxidative stress suggesting that increased rates of FAO are detrimental to cardiac function (Figure 3).85,88,89 As discussed above, one mechanism for the undesirable effect of high FAO is the lower O2 efficiency,58,59 as well as the increased presence of fatty acid derivatives that may further reduce the efficiency by uncoupling the mitochondria.90 In skeletal muscle, increased influx of fatty acid to mitochondria was associated with incomplete oxidation and development of insulin resistance.91 However, this was not observed in the mouse heart during high fat feeding92 or with increased import of long chain fatty acids to the mitochondria due to deletion of acetyl-CoA carboxylase 2 (ACC2).76
A unique aspect of cardiac metabolism in obesity and diabetes is that the supply of substrates exceeds the need for ATP synthesis. Despite increased FAO, hearts of obese and diabetic individuals accumulate a significant amount of lipid (Figure 3). A positive correlation of cardiac lipid accumulation and cardiac dysfunction has been shown giving rise to the term “lipotoxic cardiomyopathy”.93-95 Additional studies show that increases of lipid supply in animal models of cardiac lipotoxicity exceed the increases in the rate of oxidation, which eventually leads to downregulation of FAO, accumulation of toxic lipid intermediates, and contractile failure.95,96 Genetic manipulations in mice that reduce fatty acid uptake or increase the storage capacity of neutral lipids in the heart rescue the lipotoxic phenotype.45,97,98 These results suggest that the metabolic derangements in lipotoxic cardiomyopathy are rooted in the inappropriate matching of lipid supply and oxidation rather than a simple increase of FAO. The molecular mediator(s) of the cardiomyopathy in this case are largely elusive and likely multifactorial in nature. Although the accumulation of neutral lipids correlates closely with functional phenotype whether it is the cause or a mere reporter of lipotoxicity is not clear. Increases in intramuscular lipid are not always associated with detrimental effects. Both animals and humans increase triglyceride content in the heart and skeletal muscle in response to exercise training, which is associated with improved function.45,99,100
Although glucose uptake and utilization for ATP synthesis is reduced, due to insulin resistance and increased FAO, increased flux of the accessary pathways of glucose metabolism has been identified in the diabetic myocardium. In the polyol pathway, increased AR gene expression was observed while AR inhibition improved cardiac function in diabetic patients.25 In models of diabetic cardiomyoapathy, elevated levels of UDPGlcNAc, O-GlcNAc, and OGT were associated with impaired EC coupling suggesting a role of increased HBP flux in diabetic cardiac dysfunction.63,101,102 Consistent with these observations, increased expression of O-GlcNAase, the antagonist of OGT, improved cardiac function in diabetic mice.103
Metabolic Therapies for Heart Failure
Targeting substrate preference
The shift of substrate preference to glucose in pathological hypertrophy was considered adaptive based on the theoretical higher oxygen efficiency of ATP synthesis from glucose.104 Therefore, various metabolic therapies focusing on the promotion of glucose oxidation have been utilized (Table 2). One strategic target has been mCPT1, the enzyme that is the gateway for long chain fatty acid uptake into the mitochondria. The mCPT1 inhibitors, such as etomoxir, perhexiline, and oxfenicine, have been associated with reduced cardiac FAO and elevated glucose oxidation in both animal models and humans. Etomoxir has been shown to increase expression and activity of the sarcoendoplasmic reticulum (SR) calcium ATPase (SERCA).105,106 Long term treatment with etomoxir in pressure overloaded hearts improved functional capacity and myocardial performance.107 The first human clinical trial evaluating etomoxir in patients with chronic heart failure showed improved stroke volume and ejection fraction (EF).108 A clinical trial evaluated the effect of perhexiline in heart failure patients and observed improved VO2max, EF, and tolerance to dobutamine stress.109 In hypertrophic cardiomyopathy, perhexiline, in conjunction with medical management, increased the PCr/ATP ratio, corrected energy dependent LV diastolic relaxation, increased VO2, and improved quality of life.110 Although not available for human use, oxfenicine treatment in pacing-induced heart failure in dogs, when provided early, slowed the development of heart failure, prevented LV chamber dilation and LV wall thinning compared to placebo.111
Table 2. Metabolic Therapies Used in the Treatment of Heart Failure.
Italics indicate therapies with reported adverse effects. mCPT1, muscle form of carnitine palmitoyl transferase 1; PDK, pyruvate dehydrogenase kinase; MCD, malonyl CoA decarboxylase; PPAR, peroxisome proliferator-activated receptor; PDE, phosphodiesterase; AMPK, AMP-activated protein kinase; MitoQ, mitochondrial-targeted antioxidant; MitoTEMPO, Mitochondria-targeted antioxidant with superoxide and alkyl radical scavenging properties; EUK-8, superoxide dismutase and catalase mimetic; SS peptide, Szeto-Schiller peptide; PUFAs, polyunsaturated fatty acids.
| Substrate Preference: |
| mCPT1 Inhibitors |
| Partial β-oxidation inhibitors |
| PDK Inhibitors |
| MCD inhibitors |
| Nicotinic Acid Derivatives |
| PPAR Agonists |
| Insulin Sensitivity: |
| Glucagon Like Peptides (GLP-1) |
| Metformin |
| Thiazolidinediones |
| Mitochondrial Function: |
| PDE inhibitors |
| AMPK Activators |
| MitoQ |
| MitoTEMPO |
| EUK-8 |
| SS peptides |
| Dietary Modulation: |
| PUFAs |
| Vitamin E |
Dichloroacetate (DCA) increases PDH activity by inhibiting PDK, and as a consequence promotes glucose oxidation. The efficacy of DCA treatment in functional recovery during reperfusion has been shown in multiple animal models.112-115 DCA also improves cardiac function in right ventricular hypertrophy and failure.116,117 In a recent study examining hyperthyroidism and cardiac hypertrophy in rats, DCA administration completely reversed reductions in PDH flux, and significantly reduced cardiac hypertrophy without affecting cardiac output.118 Although human data are limited due to the chronic neurotoxicity of DCA,119-121 one study in patients with angina and coronary artery disease revealed that infusion of DCA during left heart catheterization was associated with increased stroke volume and myocardial efficiency index (LV work/myocardial oxygen consumption).122
Malonyl CoA decarboxylase (MCD) is a key regulator of malonyl CoA degradation and, thus, its activity relieves the inhibition of fatty acid entry into the mitochondria. Pharmacological inhibition or cardiac-specific deletion of MCD has been shown to limit FAO, increase glucose oxidation and improve cardiac function after ischemia/reperfusion injury in both rodent 123,124 and porcine models.125 Although clearly effective in treatment of cardiac ischemia, it has not been shown whether targeted inhibition of MCD in heart failure is likewise protective.
While enhancing glucose utilization appears to be beneficial for the failing heart, decreasing fatty acid supply to hypertrophied and failing hearts seems to be detrimental. Acipimox is a nicotinic acid derivative that acutely inhibits lipolysis in adipose tissue and hence decreases plasma free fatty acids (FFA) level. When administered to patients with idiopathic dilated cardiomyopathy, myocardial FFA uptake was reduced by >80% with enhanced glucose uptake. Unfortunately, cardiac work and efficiency declined after acipimox treatment.126 In long term treatment of heart failure patients with acipimox, increases in whole body glucose utilization and decreased lipid utilization rates were noted, but myocardial function, exercise capacity, and cardiac index scores remained unaffected.127 These studies suggest that promoting glucose utilization via restriction of fatty acid delivery to the myocardium is not an ideal strategy for enhancement of cardiac function via the optimization of cardiac metabolism.
As oxidation of fatty acids is the predominant and critical energy source for cardiac function, promotion of cardiac FAO would seem to be desirable for long-term treatment. Targeting PPARα, the major regulator of cardiac lipid metabolism, however, has yielded mixed outcomes. Overall, in various animal models of cardiac hypertrophy and heart failure, PPARα agonism maintained expression of genes involved in FAO with significantly improved,128,129 relatively modest,130,131 or no benefit on cardiac function.52 Furthermore, PPARα agonism has been shown to exacerbate post-ischemic injury.132,133
Activation of PPARα-mediated transcription has broad effects on lipid metabolism including lipid uptake. Excessive fatty acid uptake relative to the oxidation would contribute to lipotoxicity.97 In this regard, direct activation of FAO at the level of the mitochondria may provide a more effective therapeutic strategy for sustaining myocardial energetics. Although no drug is available for clinical studies, several proof-of concept studies have been performed in mice. Overexpression of PDK4 in mice promoted cardiac FAO at the expense of glucose but had no effect on cardiac function either under normal or ischemic conditions.134 However, introduction of the PDK4 transgene into mice expressing a constitutively active form of the phosphatase calcineurin failed to rescue cardiac dysfunction and led to an increase in mortality.135 In contrary, deletion of ACC2 increased myocardial FAO in normal mice and prevented the switch to increased glucose reliance during pressure overload induced hypertrophy.76 Cardiac function and myocardial energetics were also sustained suggesting a benefit of maintaining FAO during pathological hypertrophy. Several recent studies have also demonstrated the effectiveness of high fat diets in protection against the development of heart failure in animal models.74,136,137
Taken together, the evidence thus far suggests that enhancing glucose utilization in the hypertrophied and failing heart improves cardiac function and symptoms of heart failure in the short term. Clinical application of metabolic therapy of this kind depends on the ultimate test of its impact on the long-term mortality. However, strategies of enhancing glucose utilization by removing the contribution of fatty acids appear to be less promising. Moreover, recent preclinical studies suggest that sustaining FAO in the hypertrophied heart may be suitable for the preservation of myocardial energetics and function.74,76,137
Targeting insulin sensitivity
Insulin resistance has been shown to precede and predict the development of heart failure, independent of established diabetes.138 Moreover, insulin resistance is positively correlated with NYHA functional class.139 Since glucose uptake in the adult heart is largely controlled by insulin-sensitive mechanisms, insulin resistance would be an obstacle for measures that seek to enhance myocardial glucose utilization. Although not directly tested, insulin sensitizing agents have been used in heart failure patients with metabolic disturbances (Table 2) and have yielded auspicious results.
Thiazolidinediones (TZD), PPAR gamma agonists including rosiglitazone and pioglitazone, are used as oral hypoglycemic and insulin sensitizing agents. TZDs successfully enhance glucose uptake and oxidation, especially in diabetic animal models, and improve functional recovery after ischemia.140,141 However, one study showed that rosiglitazone increased mortality post-MI in rats without alterations in LV remodeling.142 Similarly, rosiglitazone was associated with a higher risk of cardiovascular events including congestive heart failure in the ADOPT trial, as compared to cohorts treated with glyburide or metformin.143 In the PROACTIVE trial, pioglitazone decreased all-cause mortality, non-fatal MI and stroke, but significantly increased rates of symptomatic edema and CHF in patients with diabetes and cardiovascular disease.144
Another widely used insulin-sensitizing drug is metformin that is often used as first line therapy for diabetics. Metformin acts as an AMPK activator in the liver145 and has been shown to increase glucose uptake both in basal and insulin-stimulated conditions in insulin resistant cardiomyocytes.146 Of note, activation of AMPK by metformin in human heart has not been reported. In animal studies, metformin improved left ventricular function and remodeling while reducing myocardial lipid accumulation and fibrosis.147,148 Masoudi et al. performed a retrospective cohort analysis on subjects with congestive heart failure and diabetes and found that metformin use for 1 year was associated with a 13% lower mortality compared to sulfonylurea or insulin therapy.149 Although these results are promising, randomized perspective trials are still needed to evaluate the potential clinical benefits of metformin in heart failure patients with and without diabetes.
Glucagon like peptide-1 (GLP-1) is secreted by intestinal cells in response to the presence of nutrients. Once in the circulation, it stimulates insulin secretion, enhances insulin sensitivity, and promotes glucose utilization in the myocardium. In a canine model of pacing-induced dilated cardiomyopathy, GLP-1 treatment increased myocardial glucose uptake and was associated with decreased LV end-diastolic pressure, increased stroke volume, and increased cardiac output.150 In heart failure patients, GLP-1, in addition to standard medical therapy, led to improvements in EF and maximal aerobic capacity compared with controls who received standard medical therapy alone.151 A study examining patients with an acute myocardial infarction and EF <40% after successful angioplasty treated with 72 hours of GLP-1 infusion demonstrated significantly greater EF associated with improved global and regional wall motion score indices compared to controls.152
Targeting mitochondrial function
It is well known that heart failure is associated with mitochondrial dysfunction but therapies specifically targeted to improving mitochondrial function are rather limited. The nitric oxide pathway is a potential stimulator of mitochondrial biogenesis.153 Modulation of this pathway with phosphodiesterase 5 inhibitors (PDE5I) was related to increased mitochondrial biogenesis.154 Treatment with the PDE5I, sildenafil, improved cardiac index and right ventricular EF in heart failure patients155 but additional studies are required to determine whether the benefit can be attributed to increased mitochondrial biogenesis and function.
Mitochondrial dysfunction in heart failure is associated with increased oxidative stress making mitochondria-targeted ROS scavenging an attractive therapeutic strategy. Several antioxidants that accumulate in the mitochondrial matrix have demonstrated cardioprotective effects in animal models. Mitoquinone (MitoQ) improved functional recovery from ischemia in the isolated rat heart156 as well as prevented doxorubicin-induced cardiac dysfunction, fibrosis, and apoptosis.157 MitoTEMPO, a superoxide and alkyl scavenger, demonstrated cardioprotective effects in hypertension and diabetes models.158,159 EUK–8, a superoxide dismutase and catalase mimetic, rescued cardiac dysfunction in genetic models of increased oxidative stress.160,161 Finally, Szeto-Schiller (SS) peptides have shown cardiac protection in guinea pig hearts subjected to ischemia-reperfusion injury and mouse models of hypertrophy and failure, in part, by reducing oxidative stress.162,163 So far, clinical studies using such a strategy are rather limited. However, a Phase IIa clinical trial on the safety and efficacy of the SS-peptide, Bendavia, on reperfusion injury is ongoing.164
While mitochondrial specific antioxidants have shown promising results for the treatment of heart failure, general antioxidants in clinical trials have not. Vitamin E, also known as alpha tocopherol, has been extensively studied in heart failure. Large clinical trials revealed that Vitamin E can actually increase the risk of developing heart failure after myocardial infarction.165 The HOPE and HOPE-TOO trials also suggested that long term vitamin E supplementation increases the risk of heart failure and heart failure exacerbations with no improvement in other cardiovascular outcomes.166 Further work is needed in order to elucidate the differences between mitochondrial-specific and general antioxidant therapy for heart failure.
Dietary strategies
The benefits of polyunsaturated fatty acids (PUFAs) in decreasing the incidence of coronary artery disease and sudden cardiac death are well accepted. PUFAs also improve various factors related to heart failure including lipid metabolism, mitochondrial function, endothelial function, and inflammation. Clinical evidence now suggests that PUFAs can prevent the development or progression of heart failure. A 12-year study following over 4700 adults found an inverse correlation between incidence of heart failure and dietary consumption of tuna and other fish, with the highest intake of dietary long-chain n-3 fatty acid offering a 37% lower risk of heart failure.167 A randomized double-blind, placebo controlled trial (GISSI-HF) showed that heart failure patients treated with low dose eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) for a median time of 3.9 years had a significantly lower mortality rate and decreased hospital admissions for cardiovascular causes.168 A recent meta-analysis involving 7 trials found that fish oil supplementation in non-ischemic heart failure significantly increased left ventricular EF.169 In contrary, high levels of long chain monounsaturated fatty acids (LCMUFA) were associated with a greater incidence of congestive heart failure, suggesting potential cardiac toxicity of this lipid species.170
Metabolic Modulation of Growth and Survival Pathways
The effects of metabolism on growth, proliferation, and survival pathways have been increasingly recognized in recent years, especially in cancer biology. Although a large fraction of the metabolic fluxes in the heart is devoted to oxidative metabolism for ATP synthesis, substrate metabolism has significant impact on multiple aspects of cardiac biology. Closely related to the topic of cardiac hypertrophy and failure discussed above, here we present the recent development on the role of metabolism in the regulation of cardiomyocyte growth, survival and autophagy (Figure 4).
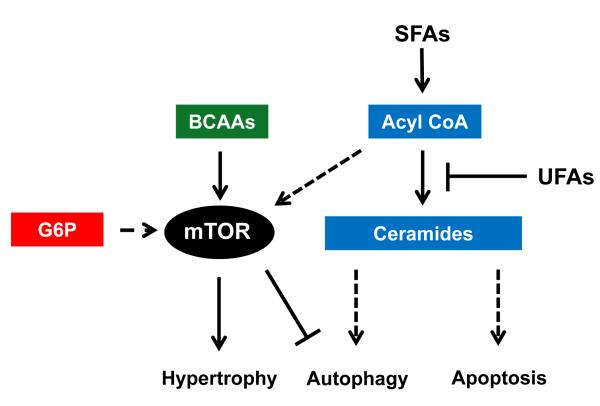
Figure 4. Metabolic Modulation of Growth and Survival Pathways.
Interactions of lipids (blue), amino acids (green), and glucose (red) metabolism with pathways of hypertrophy, autophagy, and apoptosis as represented in the literature from various cell culture and animal models. SFAs, saturated fatty acids; UFAs, unsaturated fatty acids; BCAAs, branched chain amino acids; G6P, glucose 6-phosphate; mTOR, Mammalian Target of Rapamycin.
Regulation of mTOR signaling
Recently, an interesting finding regarding the influence of fatty acids on cardiac myocyte growth was made in Burmese pythons.171 Elevations in three fatty acids (palmitic acid, myristic acid, and palmitoleic acid) were identified as inducers of reversible cardiac hypertrophy during the feeding period and were associated with increased mammalian Target of Rapamycin (mTOR) phosphorylation. This effect of fatty acids on mTOR has been previously shown in skeletal muscle and liver tissue of rats fed a high fat diet.172,173 In addition, incubation of myotubes with palmitate increased phosphorylation of S6 kinase (S6K), a downstream target of mTOR.173 Transgenic mouse models of enhanced lipid metabolism are generally associated with cardiac hypertrophy,95,96,174,175 but whether the increased uptake of fatty acids contribute to cardiac growth via the mTOR pathway has not been determined.
It has been shown that glucose phosphorylation is required for insulin-dependent activation of mTOR in the heart.176 A recent study suggested that accumulation of glucose 6-phosphate during mechanical overload activated mTOR and caused contractile dysfunction by triggering ER stress.177 Since glucose metabolism is increased in the hypertrophied and failing heart it is tempting to hypothesize that altered glucose metabolism is causally linked to the development of hypertrophy and dysfunction.
The effects of amino acids on protein synthesis have been well-studied in cultured cells, animal models, and humans.178-183 Although the exact mechanisms are not known, the presence of amino acids have been shown to activate the mTOR complex and its downstream effectors.180,182 It has been suggested that amino acids activate mTOR through a calcium dependent mechanism involving class III PI3K, or hVps34, which would combine the interaction of protein synthesis with inhibition of autophagy.181 Previous work demonstrated that mTOR and its downstream targets are affected by the availability of intracellular amino acids.184 Additional studies have specifically implicated the branched chain amino acid (BCAA), leucine, in the stimulation of the mTOR pathway.178,185,186 This has been particularly important in accounting for increased skeletal muscle protein synthesis during the post-exercise recovery period 183,187 and in atrophy associated with aging.188,189
The above evidence offers strong support to the notion that mTOR behaves as a “nutrient sensor”. It remains to be determined whether one or multiple metabolites of the aforementioned substrates have a direct binding affinity for the mTOR complex. It is also not known whether different metabolic pathways affect mTOR signaling through distinct mechanisms or via a unifying effector.
Apoptosis and autophagy
Ceramides, a sphingolipid composed of sphingosine and a fatty acid, have been purported to function as a signal which triggers apoptosis in lipotoxic cardiomyopathy.190 Elevated ceramide levels were found in the hearts of mice overexpressing enzymes of lipid metabolism, including acyl CoA synthetase (ACS),45,96 lipoprotein lipase (LPL),174 and PPARγ175, which was associated with increased apoptosis and/or cardiac dysfunction. However, high fat feeding in rodent models have not consistently recapitulated this observation. Although content of cardiac ceramides was increased in rats fed a high fat diet, no evidence of apoptosis191 or cardiac dysfunction192 was found. In addition, 10 or 12 weeks of a high fat diet fed to mice failed to significantly increase ceramide levels.193,194 Ceramides were elevated in rat hearts subjected to coronary artery ligation but provision of a high fat diet during that interval did not further increase ceramide content.131
In studies using cultured cells195-197 or neonatal rat ventricular myocytes (NRVM),198,199 addition of palmitate to the media significantly increased measures of apoptosis. Similar findings were observed with addition of stearate, suggesting long chain saturated fatty acids as the culprit.197,198 Interestingly, co-incubation with the unsaturated fatty acid, oleate, significantly reduced apoptosis measures in cells.195,198,199 Since the metabolic rate of quiescent cells is vastly different from that of the beating heart, the observation could be confounded by the low oxidation rate of fatty acids in cell culture. However, cells exposed to different species of fatty acids showed differential outcomes suggesting that the chain length and/or the degree of saturation influence the survival of cardiomyocytes under conditions of lipid overload. In rodents fed a high fat diet, a lower ceramide content and reduced apoptotic events were observed in cardiomyocytes from the group receiving predominantly unsaturated versus saturated fatty acids.137 These studies suggest that elevated cytosolic levels of palmitate are associated with increases in lipid species that have the potential to promote apoptosis. It is also suggested that oleate facilitates palmitate accumulation into the TAG pool,195 and provision of unsaturated fats in conjunction with saturated fats could promote survival by attenuation of apoptosis.
Recent work in mice demonstrated that a high milk fat based diet resulted in elevated supply of the 14 carbon (C14) saturated fat, myristic acid, which increased the presence of C14-ceramide, and was associated with cardiac hypertrophy, dysfunction, and increased autophagy.200 However, other studies using high fat feeding models in mice have suggested that autophagy is impaired during the lipid overload condition.201,202 Furthermore, hearts from a porcine model exposed to a high fat or atherogenic diet revealed progressive decreases in autophagy combined with increases in apoptosis.203 Whether metabolic derangement in the heart causes cardiac injury via inhibition of autophagy is an open question. Autophagy is critical for protein quality control, cellular homeostasis and survival. However, increased autophagy can be adaptive or maladaptive to cardiac pathologies depending on the circumstances (see for recent reviews204-208). While autophagy is known as an evolutionarily conserved response to metabolic stress, the metabolic mediators of autophagy are poorly understood at the molecular level. An expanded knowledge of the metabolic control of autophagy will facilitate targeting autophagy for therapeutics.
In summary, the knowledge on cardiac metabolism and its role in human diseases has increased explosively in recent years. Multi-disciplinary approaches in both experimental and clinical research seem to converge on the concept that the capacity and flexibility of the metabolic network is essential for cardiac function. Although energy transfer is a primary function of cardiac metabolism, the sophistication of the system is being appreciated for its regulatory role through the interactions of the ATP-producing and non-ATP producing pathways. Future advances of the field will elucidate novel disease mechanisms and identify new targets for metabolic therapy.
Acknowledgments
Sources of Funding This work is supported in part by NIH grants HL110349, HL088634 (to RT), and University of Washington Housestaff Association Research Grant, AMA Seed Grant Research Program (to SP).
Non-standard Abbreviations and Acronyms
- EF
ejection fraction
- FAO
fatty acid oxidation
- mCPT1
carnitine palmitoyl transferase 1, muscle form
- NADPH
nicotinamide adenine dinucleotide phosphate
- PDH
pyruvate dehydrogenase
- PDK4
pyruvate dehydrogenase kinase 4
- PPARα
peroxisome proliferator-activated receptor alpha
- TAG
triacylglycerol
- TCA cycle
tricarboxcylic acid cycle
Footnotes
Disclosures None
Disclaimer: The manuscript and its contents are confidential, intended for journal review purposes only, and not to be further disclosed.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 2.Opie LH. Cardiac metabolism--emergence, decline, and resurgence. Part II. Cardiovasc Res. 1992;26:817–830. doi: 10.1093/cvr/26.9.817. [DOI] [PubMed] [Google Scholar]
- 3.Opie LH. Cardiac metabolism--emergence, decline, and resurgence. Part I. Cardiovasc Res. 1992;26:721–733. doi: 10.1093/cvr/26.8.721. [DOI] [PubMed] [Google Scholar]
- 4.Schaper J, Meiser E, Stammler G. Ultrastructural morphometric analysis of myocardium from dogs, rats, hamsters, mice, and from human hearts. Circ Res. 1985;56:377–391. doi: 10.1161/01.res.56.3.377. [DOI] [PubMed] [Google Scholar]
- 5.Jeffrey FM, Diczku V, Sherry AD, Malloy CR. Substrate selection in the isolated working rat heart: effects of reperfusion, afterload, and concentration. Basic Res Cardiol. 1995;90:388–396. doi: 10.1007/BF00788500. [DOI] [PubMed] [Google Scholar]
- 6.McNulty PH, Jacob R, Deckelbaum LI, Young LH. Effect of hyperinsulinemia on myocardial amino acid uptake in patients with coronary artery disease. Metabolism. 2000;49:1365–1369. doi: 10.1053/meta.2000.9510. [DOI] [PubMed] [Google Scholar]
- 7.Wentz AE, d’Avignon DA, Weber ML, Cotter DG, Doherty JM, Kerns R, Nagarajan R, Reddy N, Sambandam N, Crawford PA. Adaptation of myocardial substrate metabolism to a ketogenic nutrient environment. J Biol Chem. 2010;285:24447–24456. doi: 10.1074/jbc.M110.100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodwin GW, Taegtmeyer H. Improved energy homeostasis of the heart in the metabolic state of exercise. Am J Physiol Heart Circ Physiol. 2000;279:H1490–1501. doi: 10.1152/ajpheart.2000.279.4.H1490. [DOI] [PubMed] [Google Scholar]
- 9.Kaijser L, Berglund B. Myocardial lactate extraction and release at rest and during heavy exercise in healthy men. Acta Physiol Scand. 1992;144:39–45. doi: 10.1111/j.1748-1716.1992.tb09265.x. [DOI] [PubMed] [Google Scholar]
- 10.Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD. beta-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Am J Physiol Heart Circ Physiol. 2003;285:H1626–1631. doi: 10.1152/ajpheart.00332.2003. [DOI] [PubMed] [Google Scholar]
- 11.Schonekess BO. Competition between lactate and fatty acids as sources of ATP in the isolated working rat heart. J Mol Cell Cardiol. 1997;29:2725–2733. doi: 10.1006/jmcc.1997.0504. [DOI] [PubMed] [Google Scholar]
- 12.Alaynick WA, Kondo RP, Xie W, He W, Dufour CR, Downes M, Jonker JW, Giles W, Naviaux RK, Giguere V, Evans RM. ERRgamma directs and maintains the transition to oxidative metabolism in the postnatal heart. Cell Metab. 2007;6:13–24. doi: 10.1016/j.cmet.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalik L, Desvergne B, Dreyer C, Gavillet M, Laurini RN, Wahli W. PPAR expression and function during vertebrate development. Int J Dev Biol. 2002;46:105–114. [PubMed] [Google Scholar]
- 15.Semenza GL. Hypoxia-inducible factor 1: regulator of mitochondrial metabolism and mediator of ischemic preconditioning. Biochim Biophys Acta. 2011;1813:1263–1268. doi: 10.1016/j.bbamcr.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugden MC, Holness MJ. Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab. 2003;284:E855–862. doi: 10.1152/ajpendo.00526.2002. [DOI] [PubMed] [Google Scholar]
- 17.Lu G, Ren S, Korge P, Choi J, Dong Y, Weiss J, Koehler C, Chen JN, Wang Y. A novel mitochondrial matrix serine/threonine protein phosphatase regulates the mitochondria permeability transition pore and is essential for cellular survival and development. Genes Dev. 2007;21:784–796. doi: 10.1101/gad.1499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu G, Sun H, She P, Youn JY, Warburton S, Ping P, Vondriska TM, Cai H, Lynch CJ, Wang Y. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. J Clin Invest. 2009;119:1678–1687. doi: 10.1172/JCI38151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zimmer HG. Regulation of and intervention into the oxidative pentose phosphate pathway and adenine nucleotide metabolism in the heart. Mol Cell Biochem. 1996:160–161. 101–109. doi: 10.1007/BF00240038. [DOI] [PubMed] [Google Scholar]
- 20.Wu G, Fang YZ, Yang S, Lupton JR, Turner ND. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 21.Jain M, Cui L, Brenner DA, Wang B, Handy DE, Leopold JA, Loscalzo J, Apstein CS, Liao R. Increased myocardial dysfunction after ischemia-reperfusion in mice lacking glucose-6-phosphate dehydrogenase. Circulation. 2004;109:898–903. doi: 10.1161/01.CIR.0000112605.43318.CA. [DOI] [PubMed] [Google Scholar]
- 22.Zimmer HG. The oxidative pentose phosphate pathway in the heart: regulation, physiological significance, and clinical implications. Basic Res Cardiol. 1992;87:303–316. doi: 10.1007/BF00796517. [DOI] [PubMed] [Google Scholar]
- 23.Doiron B, Cuif MH, Chen R, Kahn A. Transcriptional glucose signaling through the glucose response element is mediated by the pentose phosphate pathway. J Biol Chem. 1996;271:5321–5324. doi: 10.1074/jbc.271.10.5321. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura M, Uyeda K. Purification and characterization of a novel xylulose 5-phosphate-activated protein phosphatase catalyzing dephosphorylation of fructose-6-phosphate,2-kinase:fructose-2,6-bisphosphatase. J Biol Chem. 1995;270:26341–26346. doi: 10.1074/jbc.270.44.26341. [DOI] [PubMed] [Google Scholar]
- 25.Johnson BF, Nesto RW, Pfeifer MA, Slater WR, Vinik AI, Chyun DA, Law G, Wackers FJ, Young LH. Cardiac abnormalities in diabetic patients with neuropathy: effects of aldose reductase inhibitor administration. Diabetes Care. 2004;27:448–454. doi: 10.2337/diacare.27.2.448. [DOI] [PubMed] [Google Scholar]
- 26.Trueblood N, Ramasamy R. Aldose reductase inhibition improves altered glucose metabolism of isolated diabetic rat hearts. Am J Physiol. 1998;275:H75–83. doi: 10.1152/ajpheart.1998.275.1.H75. [DOI] [PubMed] [Google Scholar]
- 27.Ananthakrishnan R, Kaneko M, Hwang YC, Quadri N, Gomez T, Li Q, Caspersen C, Ramasamy R. Aldose reductase mediates myocardial ischemia-reperfusion injury in part by opening mitochondrial permeability transition pore. Am J Physiol Heart Circ Physiol. 2009;296:H333–341. doi: 10.1152/ajpheart.01012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hwang YC, Kaneko M, Bakr S, et al. Central role for aldose reductase pathway in myocardial ischemic injury. Faseb J. 2004;18:1192–1199. doi: 10.1096/fj.03-1400com. [DOI] [PubMed] [Google Scholar]
- 29.Hebert LF, Jr., Daniels MC, Zhou J, Crook ED, Turner RL, Simmons ST, Neidigh JL, Zhu JS, Baron AD, McClain DA. Overexpression of glutamine:fructose-6-phosphate amidotransferase in transgenic mice leads to insulin resistance. J Clin Invest. 1996;98:930–936. doi: 10.1172/JCI118876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wells L, Vosseller K, Hart GW. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science. 2001;291:2376–2378. doi: 10.1126/science.1058714. [DOI] [PubMed] [Google Scholar]
- 31.Pang Y, Bounelis P, Chatham JC, Marchase RB. Hexosamine pathway is responsible for inhibition by diabetes of phenylephrine-induced inotropy. Diabetes. 2004;53:1074–1081. doi: 10.2337/diabetes.53.4.1074. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Marchase RB, Chatham JC. Glutamine-induced protection of isolated rat heart from ischemia/reperfusion injury is mediated via the hexosamine biosynthesis pathway and increased protein O-GlcNAc levels. J Mol Cell Cardiol. 2007;42:177–185. doi: 10.1016/j.yjmcc.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Marchase RB, Chatham JC. Increased O-GlcNAc levels during reperfusion lead to improved functional recovery and reduced calpain proteolysis. Am J Physiol Heart Circ Physiol. 2007;293:H1391–1399. doi: 10.1152/ajpheart.00285.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J, Pang Y, Chang T, Bounelis P, Chatham JC, Marchase RB. Increased hexosamine biosynthesis and protein O-GlcNAc levels associated with myocardial protection against calcium paradox and ischemia. J Mol Cell Cardiol. 2006;40:303–312. doi: 10.1016/j.yjmcc.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin GW, Taylor CS, Taegtmeyer H. Regulation of energy metabolism of the heart during acute increase in heart work. J Biol Chem. 1998;273:29530–29539. doi: 10.1074/jbc.273.45.29530. [DOI] [PubMed] [Google Scholar]
- 36.Allard MF, Henning SL, Wambolt RB, Granleese SR, English DR, Lopaschuk GD. Glycogen metabolism in the aerobic hypertrophied rat heart. Circulation. 1997;96:676–682. doi: 10.1161/01.cir.96.2.676. [DOI] [PubMed] [Google Scholar]
- 37.Pederson BA, Chen H, Schroeder JM, Shou W, DePaoli-Roach AA, Roach PJ. Abnormal cardiac development in the absence of heart glycogen. Mol Cell Biol. 2004;24:7179–7187. doi: 10.1128/MCB.24.16.7179-7187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaefer S, Ramasamy R. Glycogen utilization and ischemic injury in the isolated rat heart. Cardiovasc Res. 1997;35:90–98. doi: 10.1016/s0008-6363(97)00087-4. [DOI] [PubMed] [Google Scholar]
- 39.Banke NH, Yan L, Pound KM, Dhar S, Reinhardt H, De Lorenzo MS, Vatner SF, Lewandowski ED. Sexual dimorphism in cardiac triacylglyceride dynamics in mice on long term caloric restriction. J Mol Cell Cardiol. 2012;52:733–740. doi: 10.1016/j.yjmcc.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carley AN, Bi J, Wang X, Banke NH, Dyck JR, O’Donnell JM, Lewandowski ED. Multiphasic triacylglycerol dynamics in the intact heart during acute in vivo overexpression of CD36. J Lipid Res. 2013;54:97–106. doi: 10.1194/jlr.M029991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Donnell JM, Fields AD, Sorokina N, Lewandowski ED. The absence of endogenous lipid oxidation in early stage heart failure exposes limits in lipid storage and turnover. J Mol Cell Cardiol. 2008;44:315–322. doi: 10.1016/j.yjmcc.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saddik M, Lopaschuk GD. Myocardial triglyceride turnover and contribution to energy substrate utilization in isolated working rat hearts. J Biol Chem. 1991;266:8162–8170. [PubMed] [Google Scholar]
- 43.O’Donnell JM, Zampino M, Alpert NM, Fasano MJ, Geenen DL, Lewandowski ED. Accelerated triacylglycerol turnover kinetics in hearts of diabetic rats include evidence for compartmented lipid storage. Am J Physiol Endocrinol Metab. 2006;290:E448–455. doi: 10.1152/ajpendo.00139.2005. [DOI] [PubMed] [Google Scholar]
- 44.Liu L, Yu S, Khan RS, Ables GP, Bharadwaj KG, Hu Y, Huggins LA, Eriksson JW, Buckett LK, Turnbull AV, Ginsberg HN, Blaner WS, Huang LS, Goldberg IJ. DGAT1 deficiency decreases PPAR expression and does not lead to lipotoxicity in cardiac and skeletal muscle. J Lipid Res. 2011;52:732–744. doi: 10.1194/jlr.M011395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Shi X, Bharadwaj KG, Ikeda S, Yamashita H, Yagyu H, Schaffer JE, Yu YH, Goldberg IJ. DGAT1 expression increases heart triglyceride content but ameliorates lipotoxicity. J Biol Chem. 2009;284:36312–36323. doi: 10.1074/jbc.M109.049817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haemmerle G, Lass A, Zimmermann R, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 47.Haemmerle G, Moustafa T, Woelkart G, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kienesberger PC, Pulinilkunnil T, Sung MM, Nagendran J, Haemmerle G, Kershaw EE, Young ME, Light PE, Oudit GY, Zechner R, Dyck JR. Myocardial ATGL Over-expression Decreases the Reliance on Fatty Acid Oxidation and Protects Against Pressure Overload-Induced Cardiac Dysfunction. Mol Cell Biol. 2012;32:740–750. doi: 10.1128/MCB.06470-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Akki A, Smith K, Seymour AM. Compensated cardiac hypertrophy is characterised by a decline in palmitate oxidation. Mol Cell Biochem. 2008;311:215–224. doi: 10.1007/s11010-008-9711-y. [DOI] [PubMed] [Google Scholar]
- 50.Allard MF, Schonekess BO, Henning SL, English DR, Lopaschuk GD. Contribution of oxidative metabolism and glycolysis to ATP production in hypertrophied hearts. Am J Physiol. 1994;267:H742–750. doi: 10.1152/ajpheart.1994.267.2.H742. [DOI] [PubMed] [Google Scholar]
- 51.Barger PM, Kelly DP. Fatty acid utilization in the hypertrophied and failing heart: molecular regulatory mechanisms. Am J Med Sci. 1999;318:36–42. doi: 10.1097/00000441-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Young ME, Laws FA, Goodwin GW, Taegtmeyer H. Reactivation of peroxisome proliferator-activated receptor alpha is associated with contractile dysfunction in hypertrophied rat heart. J Biol Chem. 2001;276:44390–44395. doi: 10.1074/jbc.M103826200. [DOI] [PubMed] [Google Scholar]
- 53.Nascimben L, Ingwall JS, Lorell BH, Pinz I, Schultz V, Tornheim K, Tian R. Mechanisms for increased glycolysis in the hypertrophied rat heart. Hypertension. 2004;44:662–667. doi: 10.1161/01.HYP.0000144292.69599.0c. [DOI] [PubMed] [Google Scholar]
- 54.Leong HS, Grist M, Parsons H, Wambolt RB, Lopaschuk GD, Brownsey R, Allard MF. Accelerated rates of glycolysis in the hypertrophied heart: are they a methodological artifact? Am J Physiol Endocrinol Metab. 2002;282:E1039–1045. doi: 10.1152/ajpendo.00507.2001. [DOI] [PubMed] [Google Scholar]
- 55.El Alaoui-Talibi Z, Guendouz A, Moravec M, Moravec J. Control of oxidative metabolism in volume-overloaded rat hearts: effect of propionyl-L-carnitine. Am J Physiol. 1997;272:H1615–1624. doi: 10.1152/ajpheart.1997.272.4.H1615. [DOI] [PubMed] [Google Scholar]
- 56.Wambolt RB, Lopaschuk GD, Brownsey RW, Allard MF. Dichloroacetate improves postischemic function of hypertrophied rat hearts. J Am Coll Cardiol. 2000;36:1378–1385. doi: 10.1016/s0735-1097(00)00856-1. [DOI] [PubMed] [Google Scholar]
- 57.Lehman JJ, Kelly DP. Gene regulatory mechanisms governing energy metabolism during cardiac hypertrophic growth. Heart Fail Rev. 2002;7:175–185. doi: 10.1023/a:1015332726303. [DOI] [PubMed] [Google Scholar]
- 58.Burkhoff D, Weiss RG, Schulman SP, Kalil-Filho R, Wannenburg T, Gerstenblith G. Influence of metabolic substrate on rat heart function and metabolism at different coronary flows. Am J Physiol. 1991;261:H741–750. doi: 10.1152/ajpheart.1991.261.3.H741. [DOI] [PubMed] [Google Scholar]
- 59.Korvald C, Elvenes OP, Myrmel T. Myocardial substrate metabolism influences left ventricular energetics in vivo. Am J Physiol Heart Circ Physiol. 2000;278:H1345–1351. doi: 10.1152/ajpheart.2000.278.4.H1345. [DOI] [PubMed] [Google Scholar]
- 60.Davidoff AJ, Davidson MB, Carmody MW, Davis ME, Ren J. Diabetic cardiomyocyte dysfunction and myocyte insulin resistance: role of glucose-induced PKC activity. Mol Cell Biochem. 2004;262:155–163. doi: 10.1023/b:mcbi.0000038231.68078.4b. [DOI] [PubMed] [Google Scholar]
- 61.Makino A, Suarez J, Gawlowski T, Han W, Wang H, Scott BT, Dillmann WH. Regulation of mitochondrial morphology and function by O-GlcNAcylation in neonatal cardiac myocytes. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1296–1302. doi: 10.1152/ajpregu.00437.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ren J, Gintant GA, Miller RE, Davidoff AJ. High extracellular glucose impairs cardiac EC coupling in a glycosylation-dependent manner. Am J Physiol. 1997;273:H2876–2883. doi: 10.1152/ajpheart.1997.273.6.H2876. [DOI] [PubMed] [Google Scholar]
- 63.Suarez J, Hu Y, Makino A, Fricovsky E, Wang H, Dillmann WH. Alterations in mitochondrial function and cytosolic calcium induced by hyperglycemia are restored by mitochondrial transcription factor A in cardiomyocytes. Am J Physiol Cell Physiol. 2008;295:C1561–1568. doi: 10.1152/ajpcell.00076.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luptak I, Yan J, Cui L, Jain M, Liao R, Tian R. Long-term effects of increased glucose entry on mouse hearts during normal aging and ischemic stress. Circulation. 2007;116:901–909. doi: 10.1161/CIRCULATIONAHA.107.691253. [DOI] [PubMed] [Google Scholar]
- 65.Liao R, Nascimben L, Friedrich J, Gwathmey JK, Ingwall JS. Decreased energy reserve in an animal model of dilated cardiomyopathy. Relationship to contractile performance. Circ Res. 1996;78:893–902. doi: 10.1161/01.res.78.5.893. [DOI] [PubMed] [Google Scholar]
- 66.Tian R, Nascimben L, Ingwall JS, Lorell BH. Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation. 1997;96:1313–1319. doi: 10.1161/01.cir.96.4.1313. [DOI] [PubMed] [Google Scholar]
- 67.Ingwall JS. Energy metabolism in heart failure and remodelling. Cardiovasc Res. 2009;81:412–419. doi: 10.1093/cvr/cvn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Neubauer S. The failing heart--an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 69.Neubauer S, Horn M, Cramer M, Harre K, Newell JB, Peters W, Pabst T, Ertl G, Hahn D, Ingwall JS, Kochsiek K. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96:2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 70.Ingwall JS, Weiss RG. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ Res. 2004;95:135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 71.Nikolaidis LA, Sturzu A, Stolarski C, Elahi D, Shen YT, Shannon RP. The development of myocardial insulin resistance in conscious dogs with advanced dilated cardiomyopathy. Cardiovasc Res. 2004;61:297–306. doi: 10.1016/j.cardiores.2003.11.027. [DOI] [PubMed] [Google Scholar]
- 72.Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, Stevenson JC, Coats AJ. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. J Am Coll Cardiol. 1997;30:527–532. doi: 10.1016/s0735-1097(97)00185-x. [DOI] [PubMed] [Google Scholar]
- 73.Liao R, Jain M, Cui L, D’Agostino J, Aiello F, Luptak I, Ngoy S, Mortensen RM, Tian R. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Circulation. 2002;106:2125–2131. doi: 10.1161/01.cir.0000034049.61181.f3. [DOI] [PubMed] [Google Scholar]
- 74.Chess DJ, Khairallah RJ, O’Shea KM, Xu W, Stanley WC. A high-fat diet increases adiposity but maintains mitochondrial oxidative enzymes without affecting development of heart failure with pressure overload. Am J Physiol Heart Circ Physiol. 2009;297:H1585–1593. doi: 10.1152/ajpheart.00599.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta A, Akki A, Wang Y, et al. Creatine kinase-mediated improvement of function in failing mouse hearts provides causal evidence the failing heart is energy starved. J Clin Invest. 2012;122:291–302. doi: 10.1172/JCI57426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolwicz SC, Jr., Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Circ Res. 2012;111:728–738. doi: 10.1161/CIRCRESAHA.112.268128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pound KM, Sorokina N, Ballal K, Berkich DA, Fasano M, Lanoue KF, Taegtmeyer H, O’Donnell JM, Lewandowski ED. Substrate-enzyme competition attenuates upregulated anaplerotic flux through malic enzyme in hypertrophied rat heart and restores triacylglyceride content: attenuating upregulated anaplerosis in hypertrophy. Circ Res. 2009;104:805–812. doi: 10.1161/CIRCRESAHA.108.189951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorokina N, O’Donnell JM, McKinney RD, Pound KM, Woldegiorgis G, LaNoue KF, Ballal K, Taegtmeyer H, Buttrick PM, Lewandowski ED. Recruitment of compensatory pathways to sustain oxidative flux with reduced carnitine palmitoyltransferase I activity characterizes inefficiency in energy metabolism in hypertrophied hearts. Circulation. 2007;115:2033–2041. doi: 10.1161/CIRCULATIONAHA.106.668665. [DOI] [PubMed] [Google Scholar]
- 79.Gupte RS, Vijay V, Marks B, Levine RJ, Sabbah HN, Wolin MS, Recchia FA, Gupte SA. Upregulation of glucose-6-phosphate dehydrogenase and NAD(P)H oxidase activity increases oxidative stress in failing human heart. J Card Fail. 2007;13:497–506. doi: 10.1016/j.cardfail.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 80.Gupte SA, Levine RJ, Gupte RS, Young ME, Lionetti V, Labinskyy V, Floyd BC, Ojaimi C, Bellomo M, Wolin MS, Recchia FA. Glucose-6-phosphate dehydrogenase-derived NADPH fuels superoxide production in the failing heart. J Mol Cell Cardiol. 2006;41:340–349. doi: 10.1016/j.yjmcc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Hecker PA, Lionetti V, Ribeiro RF, Jr., Rastogi S, Brown BH, O’Connell KA, Cox JW, Shekar KC, Gamble DM, Sabbah HN, Leopold JA, Gupte SA, Recchia FA, Stanley WC. Glucose 6-phosphate dehydrogenase deficiency increases redox stress and moderately accelerates the development of heart failure. Circ Heart Fail. 2013;6:118–126. doi: 10.1161/CIRCHEARTFAILURE.112.969576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Young ME, Yan J, Razeghi P, Cooksey RC, Guthrie PH, Stepkowski SM, McClain DA, Tian R, Taegtmeyer H. Proposed regulation of gene expression by glucose in rodent heart. Gene Regul Syst Bio. 2007;1:251–262. doi: 10.4137/grsb.s222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhao S, Xu W, Jiang W, et al. Regulation of cellular metabolism by protein lysine acetylation. Science. 2010;327:1000–1004. doi: 10.1126/science.1179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belke DD, Larsen TS, Gibbs EM, Severson DL. Altered metabolism causes cardiac dysfunction in perfused hearts from diabetic (db/db) mice. Am J Physiol Endocrinol Metab. 2000;279:E1104–1113. doi: 10.1152/ajpendo.2000.279.5.E1104. [DOI] [PubMed] [Google Scholar]
- 86.Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, Cooksey RC, Litwin SE, Abel ED. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology. 2005;146:5341–5349. doi: 10.1210/en.2005-0938. [DOI] [PubMed] [Google Scholar]
- 87.Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–1532. doi: 10.1016/j.jacc.2009.04.074. [DOI] [PubMed] [Google Scholar]
- 88.Boudina S, Sena S, Theobald H, Sheng X, Wright JJ, Hu XX, Aziz S, Johnson JI, Bugger H, Zaha VG, Abel ED. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 89.Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–1446. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- 90.Cole MA, Murray AJ, Cochlin LE, Heather LC, McAleese S, Knight NS, Sutton E, Jamil AA, Parassol N, Clarke K. A high fat diet increases mitochondrial fatty acid oxidation and uncoupling to decrease efficiency in rat heart. Basic Res Cardiol. 2011;106:447–457. doi: 10.1007/s00395-011-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 92.Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JR, Muoio DM, Lopaschuk GD. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet-induced obese mice lacking malonyl CoA decarboxylase. Diabetes. 2009;58:1766–1775. doi: 10.2337/db09-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni-D’Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. doi: 10.1002/mrm.10372. [DOI] [PubMed] [Google Scholar]
- 94.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but not gone: the rediscovery of fatty heart, the most common unrecognized disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 95.Finck BN, Lehman JJ, Leone TC, Welch MJ, Bennett MJ, Kovacs A, Han X, Gross RW, Kozak R, Lopaschuk GD, Kelly DP. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chiu HC, Kovacs A, Ford DA, Hsu FF, Garcia R, Herrero P, Saffitz JE, Schaffer JE. A novel mouse model of lipotoxic cardiomyopathy. J Clin Invest. 2001;107:813–822. doi: 10.1172/JCI10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang J, Sambandam N, Han X, Gross RW, Courtois M, Kovacs A, Febbraio M, Finck BN, Kelly DP. CD36 deficiency rescues lipotoxic cardiomyopathy. Circ Res. 2007;100:1208–1217. doi: 10.1161/01.RES.0000264104.25265.b6. [DOI] [PubMed] [Google Scholar]
- 98.Yu X, Burgess SC, Ge H, Wong KK, Nassem RH, Garry DJ, Sherry AD, Malloy CR, Berger JP, Li C. Inhibition of cardiac lipoprotein utilization by transgenic overexpression of Angptl4 in the heart. Proc Natl Acad Sci U S A. 2005;102:1767–1772. doi: 10.1073/pnas.0409564102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ikeda S, Miyazaki H, Nakatani T, Kai Y, Kamei Y, Miura S, Tsuboyama-Kasaoka N, Ezaki O. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun. 2002;296:395–400. doi: 10.1016/s0006-291x(02)00883-5. [DOI] [PubMed] [Google Scholar]
- 100.Schenk S, Horowitz JF. Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J Clin Invest. 2007;117:1690–1698. doi: 10.1172/JCI30566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fulop N, Mason MM, Dutta K, Wang P, Davidoff AJ, Marchase RB, Chatham JC. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol. 2007;292:C1370–1378. doi: 10.1152/ajpcell.00422.2006. [DOI] [PubMed] [Google Scholar]
- 102.Fricovsky ES, Suarez J, Ihm SH, Scott BT, Suarez-Ramirez JA, Banerjee I, Torres-Gonzalez M, Wang H, Ellrott I, Maya-Ramos L, Villarreal F, Dillmann WH. Excess protein O-GlcNAcylation and the progression of diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol. 2012;303:R689–699. doi: 10.1152/ajpregu.00548.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hu Y, Belke D, Suarez J, Swanson E, Clark R, Hoshijima M, Dillmann WH. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–1013. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 104.Opie LH. Heart physiology : from cell to circulation. 4th ed Lippincott Williams & Wilkins; Philadelphia: 2004. [Google Scholar]
- 105.Rupp H, Wahl R, Hansen M. Influence of diet and carnitine palmitoyltransferase I inhibition on myosin and sarcoplasmic reticulum. J Appl Physiol. 1992;72:352–360. doi: 10.1152/jappl.1992.72.1.352. [DOI] [PubMed] [Google Scholar]
- 106.Zarain-Herzberg A, Rupp H, Elimban V, Dhalla NS. Modification of sarcoplasmic reticulum gene expression in pressure overload cardiac hypertrophy by etomoxir. Faseb J. 1996;10:1303–1309. doi: 10.1096/fasebj.10.11.8836044. [DOI] [PubMed] [Google Scholar]
- 107.Turcani M, Rupp H. Etomoxir improves left ventricular performance of pressure-overloaded rat heart. Circulation. 1997;96:3681–3686. doi: 10.1161/01.cir.96.10.3681. [DOI] [PubMed] [Google Scholar]
- 108.Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci (Lond) 2000;99:27–35. [PubMed] [Google Scholar]
- 109.Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, Ashrafian H, Horowitz J, Fraser AG, Clarke K, Frenneaux M. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Circulation. 2005;112:3280–3288. doi: 10.1161/CIRCULATIONAHA.105.551457. [DOI] [PubMed] [Google Scholar]
- 110.Abozguia K, Elliott P, McKenna W, Phan TT, Nallur-Shivu G, Ahmed I, Maher AR, Kaur K, Taylor J, Henning A, Ashrafian H, Watkins H, Frenneaux M. Metabolic modulator perhexiline corrects energy deficiency and improves exercise capacity in symptomatic hypertrophic cardiomyopathy. Circulation. 2010;122:1562–1569. doi: 10.1161/CIRCULATIONAHA.109.934059. [DOI] [PubMed] [Google Scholar]
- 111.Lionetti V, Linke A, Chandler MP, Young ME, Penn MS, Gupte S, d’Agostino C, Hintze TH, Stanley WC, Recchia FA. Carnitine palmitoyl transferase-I inhibition prevents ventricular remodeling and delays decompensation in pacing-induced heart failure. Cardiovasc Res. 2005;66:454–461. doi: 10.1016/j.cardiores.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 112.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Circulation. 1995;91:2071–2079. doi: 10.1161/01.cir.91.7.2071. [DOI] [PubMed] [Google Scholar]
- 113.Kudej RK, White LT, Kudej AB, Vatner SF, Lewandowski ED. Brief increase in carbohydrate oxidation after reperfusion reverses myocardial stunning in conscious pigs. Circulation. 2002;106:2836–2841. doi: 10.1161/01.cir.0000039326.87475.98. [DOI] [PubMed] [Google Scholar]
- 114.Churchill EN, Murriel CL, Chen CH, Mochly-Rosen D, Szweda LI. Reperfusion-induced translocation of deltaPKC to cardiac mitochondria prevents pyruvate dehydrogenase reactivation. Circ Res. 2005;97:78–85. doi: 10.1161/01.RES.0000173896.32522.6e. [DOI] [PubMed] [Google Scholar]
- 115.McVeigh JJ, Lopaschuk GD. Dichloroacetate stimulation of glucose oxidation improves recovery of ischemic rat hearts. Am J Physiol. 1990;259:H1079–1085. doi: 10.1152/ajpheart.1990.259.4.H1079. [DOI] [PubMed] [Google Scholar]
- 116.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med (Berl) 2010;88:1011–1020. doi: 10.1007/s00109-010-0679-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Atherton HJ, Dodd MS, Heather LC, Schroeder MA, Griffin JL, Radda GK, Clarke K, Tyler DJ. Role of pyruvate dehydrogenase inhibition in the development of hypertrophy in the hyperthyroid rat heart: a combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation. 2011;123:2552–2561. doi: 10.1161/CIRCULATIONAHA.110.011387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kurlemann G, Paetzke I, Moller H, Masur H, Schuierer G, Weglage J, Koch HG. Therapy of complex I deficiency: peripheral neuropathy during dichloroacetate therapy. Eur J Pediatr. 1995;154:928–932. doi: 10.1007/BF01957508. [DOI] [PubMed] [Google Scholar]
- 120.Oishi K, Yoshioka M, Ozawa R, Yamamoto T, Oya Y, Ogawa M, Kawai M. [Dichloroacetate treatment for adult patients with mitochondrial disease] Rinsho Shinkeigaku. 2003;43:154–161. [PubMed] [Google Scholar]
- 121.Spruijt L, Naviaux RK, McGowan KA, Nyhan WL, Sheean G, Haas RH, Barshop BA. Nerve conduction changes in patients with mitochondrial diseases treated with dichloroacetate. Muscle Nerve. 2001;24:916–924. doi: 10.1002/mus.1089. [DOI] [PubMed] [Google Scholar]
- 122.Wargovich TJ, MacDonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am J Cardiol. 1988;61:65–70. doi: 10.1016/0002-9149(88)91306-9. [DOI] [PubMed] [Google Scholar]
- 123.Dyck JR, Cheng JF, Stanley WC, Barr R, Chandler MP, Brown S, Wallace D, Arrhenius T, Harmon C, Yang G, Nadzan AM, Lopaschuk GD. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res. 2004;94:e78–84. doi: 10.1161/01.RES.0000129255.19569.8f. [DOI] [PubMed] [Google Scholar]
- 124.Dyck JR, Hopkins TA, Bonnet S, Michelakis ED, Young ME, Watanabe M, Kawase Y, Jishage K, Lopaschuk GD. Absence of malonyl coenzyme A decarboxylase in mice increases cardiac glucose oxidation and protects the heart from ischemic injury. Circulation. 2006;114:1721–1728. doi: 10.1161/CIRCULATIONAHA.106.642009. [DOI] [PubMed] [Google Scholar]
- 125.Stanley WC, Morgan EE, Huang H, McElfresh TA, Sterk JP, Okere IC, Chandler MP, Cheng J, Dyck JR, Lopaschuk GD. Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Am J Physiol Heart Circ Physiol. 2005;289:H2304–2309. doi: 10.1152/ajpheart.00599.2005. [DOI] [PubMed] [Google Scholar]
- 126.Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation. 2006;114:2130–2137. doi: 10.1161/CIRCULATIONAHA.106.645184. [DOI] [PubMed] [Google Scholar]
- 127.Halbirk M, Norrelund H, Moller N, Schmitz O, Gotzsche L, Nielsen R, Nielsen-Kudsk JE, Nielsen SS, Nielsen TT, Eiskjaer H, Botker HE, Wiggers H. Suppression of circulating free fatty acids with acipimox in chronic heart failure patients changes whole body metabolism but does not affect cardiac function. Am J Physiol Heart Circ Physiol. 2010;299:H1220–1225. doi: 10.1152/ajpheart.00475.2010. [DOI] [PubMed] [Google Scholar]
- 128.Brigadeau F, Gele P, Wibaux M, Marquie C, Martin-Nizard F, Torpier G, Fruchart JC, Staels B, Duriez P, Lacroix D. The PPARalpha activator fenofibrate slows down the progression of the left ventricular dysfunction in porcine tachycardia-induced cardiomyopathy. J Cardiovasc Pharmacol. 2007;49:408–415. doi: 10.1097/FJC.0b013e3180544540. [DOI] [PubMed] [Google Scholar]
- 129.Lebrasseur NK, Duhaney TA, De Silva DS, Cui L, Ip PC, Joseph L, Sam F. Effects of fenofibrate on cardiac remodeling in aldosterone-induced hypertension. Hypertension. 2007;50:489–496. doi: 10.1161/HYPERTENSIONAHA.107.092403. [DOI] [PubMed] [Google Scholar]
- 130.Labinskyy V, Bellomo M, Chandler MP, Young ME, Lionetti V, Qanud K, Bigazzi F, Sampietro T, Stanley WC, Recchia FA. Chronic activation of peroxisome proliferator-activated receptor-alpha with fenofibrate prevents alterations in cardiac metabolic phenotype without changing the onset of decompensation in pacing-induced heart failure. J Pharmacol Exp Ther. 2007;321:165–171. doi: 10.1124/jpet.106.116871. [DOI] [PubMed] [Google Scholar]
- 131.Morgan EE, Rennison JH, Young ME, McElfresh TA, Kung TA, Tserng KY, Hoit BD, Stanley WC, Chandler MP. Effects of chronic activation of peroxisome proliferator-activated receptor-alpha or high-fat feeding in a rat infarct model of heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H1899–1904. doi: 10.1152/ajpheart.01014.2005. [DOI] [PubMed] [Google Scholar]
- 132.Hafstad AD, Khalid AM, Hagve M, Lund T, Larsen TS, Severson DL, Clarke K, Berge RK, Aasum E. Cardiac peroxisome proliferator-activated receptor-alpha activation causes increased fatty acid oxidation, reducing efficiency and post-ischaemic functional loss. Cardiovasc Res. 2009;83:519–526. doi: 10.1093/cvr/cvp132. [DOI] [PubMed] [Google Scholar]
- 133.Sambandam N, Morabito D, Wagg C, Finck BN, Kelly DP, Lopaschuk GD. Chronic activation of PPARalpha is detrimental to cardiac recovery after ischemia. Am J Physiol Heart Circ Physiol. 2006;290:H87–95. doi: 10.1152/ajpheart.00285.2005. [DOI] [PubMed] [Google Scholar]
- 134.Chambers KT, Leone TC, Sambandam N, Kovacs A, Wagg CS, Lopaschuk GD, Finck BN, Kelly DP. Chronic inhibition of pyruvate dehydrogenase in heart triggers an adaptive metabolic response. J Biol Chem. 2011;286:11155–11162. doi: 10.1074/jbc.M110.217349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao G, Jeoung NH, Burgess SC, Rosaaen-Stowe KA, Inagaki T, Latif S, Shelton JM, McAnally J, Bassel-Duby R, Harris RA, Richardson JA, Kliewer SA. Overexpression of pyruvate dehydrogenase kinase 4 in heart perturbs metabolism and exacerbates calcineurin-induced cardiomyopathy. Am J Physiol Heart Circ Physiol. 2008;294:H936–943. doi: 10.1152/ajpheart.00870.2007. [DOI] [PubMed] [Google Scholar]
- 136.Rennison JH, McElfresh TA, Chen X, Anand VR, Hoit BD, Hoppel CL, Chandler MP. Prolonged exposure to high dietary lipids is not associated with lipotoxicity in heart failure. J Mol Cell Cardiol. 2009;46:883–890. doi: 10.1016/j.yjmcc.2009.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okere IC, Young ME, McElfresh TA, Chess DJ, Sharov VG, Sabbah HN, Hoit BD, Ernsberger P, Chandler MP, Stanley WC. Low carbohydrate/high-fat diet attenuates cardiac hypertrophy, remodeling, and altered gene expression in hypertension. Hypertension. 2006;48:1116–1123. doi: 10.1161/01.HYP.0000248430.26229.0f. [DOI] [PubMed] [Google Scholar]
- 138.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin resistance and risk of congestive heart failure. Jama. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 139.Suskin N, McKelvie RS, Burns RJ, Latini R, Pericak D, Probstfield J, Rouleau JL, Sigouin C, Solymoss CB, Tsuyuki R, White M, Yusuf S. Glucose and insulin abnormalities relate to functional capacity in patients with congestive heart failure. Eur Heart J. 2000;21:1368–1375. doi: 10.1053/euhj.1999.2043. [DOI] [PubMed] [Google Scholar]
- 140.Sidell RJ, Cole MA, Draper NJ, Desrois M, Buckingham RE, Clarke K. Thiazolidinedione treatment normalizes insulin resistance and ischemic injury in the zucker Fatty rat heart. Diabetes. 2002;51:1110–1117. doi: 10.2337/diabetes.51.4.1110. [DOI] [PubMed] [Google Scholar]
- 141.Zhu P, Lu L, Xu Y, Schwartz GG. Troglitazone improves recovery of left ventricular function after regional ischemia in pigs. Circulation. 2000;101:1165–1171. doi: 10.1161/01.cir.101.10.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lygate CA, Hulbert K, Monfared M, Cole MA, Clarke K, Neubauer S. The PPARgamma-activator rosiglitazone does not alter remodeling but increases mortality in rats post-myocardial infarction. Cardiovasc Res. 2003;58:632–637. doi: 10.1016/s0008-6363(03)00289-x. [DOI] [PubMed] [Google Scholar]
- 143.Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 144.Robinson JG. Update on PPAR agonists: the clinical significance of FIELD and PROACTIVE. Curr Atheroscler Rep. 2007;9:64–71. doi: 10.1007/BF02693930. [DOI] [PubMed] [Google Scholar]
- 145.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bertrand L, Ginion A, Beauloye C, Hebert AD, Guigas B, Hue L, Vanoverschelde JL. AMPK activation restores the stimulation of glucose uptake in an in vitro model of insulin-resistant cardiomyocytes via the activation of protein kinase B. Am J Physiol Heart Circ Physiol. 2006;291:H239–250. doi: 10.1152/ajpheart.01269.2005. [DOI] [PubMed] [Google Scholar]
- 147.Wang XF, Zhang JY, Li L, Zhao XY, Tao HL, Zhang L. Metformin improves cardiac function in rats via activation of AMP-activated protein kinase. Clin Exp Pharmacol Physiol. 2011;38:94–101. doi: 10.1111/j.1440-1681.2010.05470.x. [DOI] [PubMed] [Google Scholar]
- 148.Cittadini A, Napoli R, Monti MG, Rea D, Longobardi S, Netti PA, Walser M, Sama M, Aimaretti G, Isgaard J, Sacca L. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes. 2012;61:944–953. doi: 10.2337/db11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Masoudi FA, Inzucchi SE, Wang Y, Havranek EP, Foody JM, Krumholz HM. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–590. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 150.Nikolaidis LA, Elahi D, Shen YT, Shannon RP. Active metabolite of GLP-1 mediates myocardial glucose uptake and improves left ventricular performance in conscious dogs with dilated cardiomyopathy. Am J Physiol Heart Circ Physiol. 2005;289:H2401–2408. doi: 10.1152/ajpheart.00347.2005. [DOI] [PubMed] [Google Scholar]
- 151.Sokos GG, Nikolaidis LA, Mankad S, Elahi D, Shannon RP. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–699. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 152.Nikolaidis LA, Mankad S, Sokos GG, Miske G, Shah A, Elahi D, Shannon RP. Effects of glucagon-like peptide-1 in patients with acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–965. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 153.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 154.De Toni L, Strapazzon G, Gianesello L, Caretta N, Pilon C, Bruttocao A, Foresta C. Effects of type 5-phosphodiesterase inhibition on energy metabolism and mitochondrial biogenesis in human adipose tissue ex vivo. J Endocrinol Invest. 2011;34:738–741. doi: 10.1007/BF03346724. [DOI] [PubMed] [Google Scholar]
- 155.Cvelich RG, Roberts SC, Brown JN. Phosphodiesterase type 5 inhibitors as adjunctive therapy in the management of systolic heart failure. Ann Pharmacother. 2011;45:1551–1558. doi: 10.1345/aph.1Q421. [DOI] [PubMed] [Google Scholar]
- 156.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. Faseb J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 157.Chandran K, Aggarwal D, Migrino RQ, Joseph J, McAllister D, Konorev EA, Antholine WE, Zielonka J, Srinivasan S, Avadhani NG, Kalyanaraman B. Doxorubicin inactivates myocardial cytochrome c oxidase in rats: cardioprotection by Mito-Q. Biophys J. 2009;96:1388–1398. doi: 10.1016/j.bpj.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Dikalova AE, Bikineyeva AT, Budzyn K, Nazarewicz RR, McCann L, Lewis W, Harrison DG, Dikalov SI. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luo M, Guan X, Luczak ED, et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.van Empel VP, Bertrand AT, van Oort RJ, van der Nagel R, Engelen M, van Rijen HV, Doevendans PA, Crijns HJ, Ackerman SL, Sluiter W, De Windt LJ. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol. 2006;48:824–832. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 161.Kawakami S, Matsuda A, Sunagawa T, Noda Y, Kaneko T, Tahara S, Hiraumi Y, Adachi S, Matsui H, Ando K, Fujita T, Maruyama N, Shirasawa T, Shimizu T. Antioxidant, EUK-8, prevents murine dilated cardiomyopathy. Circ J. 2009;73:2125–2134. doi: 10.1253/circj.cj-09-0204. [DOI] [PubMed] [Google Scholar]
- 162.Dai DF, Chen T, Szeto H, Nieves-Cintron M, Kutyavin V, Santana LF, Rabinovitch PS. Mitochondrial targeted antioxidant Peptide ameliorates hypertensive cardiomyopathy. J Am Coll Cardiol. 2011;58:73–82. doi: 10.1016/j.jacc.2010.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao K, Zhao GM, Wu D, Soong Y, Birk AV, Schiller PW, Szeto HH. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 164.Chakrabarti AK, Feeney K, Abueg C, Brown DA, Czyz E, Tendera M, Janosi A, Giugliano RP, Kloner RA, Weaver WD, Bode C, Godlewski J, Merkely B, Gibson CM. Rationale and design of the EMBRACE STEMI Study: A phase 2a, randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of intravenous Bendavia on reperfusion injury in patients treated with standard therapy including primary percutaneous coronary intervention and stenting for ST-segment elevation myocardial infarction. Am Heart J. 2013;165:509–514. e507. doi: 10.1016/j.ahj.2012.12.008. [DOI] [PubMed] [Google Scholar]
- 165.Marchioli R, Levantesi G, Macchia A, Marfisi RM, Nicolosi GL, Tavazzi L, Tognoni G, Valagussa F. Vitamin E increases the risk of developing heart failure after myocardial infarction: Results from the GISSI-Prevenzione trial. J Cardiovasc Med (Hagerstown) 2006;7:347–350. doi: 10.2459/01.JCM.0000223257.09062.17. [DOI] [PubMed] [Google Scholar]
- 166.Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, Ross C, Arnold A, Sleight P, Probstfield J, Dagenais GR. Effects of long-term vitamin E supplementation on cardiovascular events and cancer: a randomized controlled trial. Jama. 2005;293:1338–1347. doi: 10.1001/jama.293.11.1338. [DOI] [PubMed] [Google Scholar]
- 167.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 168.Tavazzi L, Maggioni AP, Marchioli R, Barlera S, Franzosi MG, Latini R, Lucci D, Nicolosi GL, Porcu M, Tognoni G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1223–1230. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 169.Xin W, Wei W, Li X. Effects of fish oil supplementation on cardiac function in chronic heart failure: a meta-analysis of randomised controlled trials. Heart. 2012;98:1620–1625. doi: 10.1136/heartjnl-2012-302119. [DOI] [PubMed] [Google Scholar]
- 170.Imamura F, Lemaitre RN, King IB, Song X, Steffen LM, Folsom AR, Siscovick DS, Mozaffarian D. Long-Chain Monounsaturated Fatty Acids and Incidence of Congestive Heart Failure in Two Prospective Cohorts. Circulation. 2013 doi: 10.1161/CIRCULATIONAHA.112.001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Riquelme CA, Magida JA, Harrison BC, Wall CE, Marr TG, Secor SM, Leinwand LA. Fatty acids identified in the Burmese python promote beneficial cardiac growth. Science. 2011;334:528–531. doi: 10.1126/science.1210558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 173.Rivas DA, Yaspelkis BB, 3rd, Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta-D-ribofuranoside. J Endocrinol. 2009;202:441–451. doi: 10.1677/JOE-09-0202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Son NH, Park TS, Yamashita H, Yokoyama M, Huggins LA, Okajima K, Homma S, Szabolcs MJ, Huang LS, Goldberg IJ. Cardiomyocyte expression of PPARgamma leads to cardiac dysfunction in mice. J Clin Invest. 2007;117:2791–2801. doi: 10.1172/JCI30335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharma S, Guthrie PH, Chan SS, Haq S, Taegtmeyer H. Glucose phosphorylation is required for insulin-dependent mTOR signalling in the heart. Cardiovasc Res. 2007;76:71–80. doi: 10.1016/j.cardiores.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sen S, Kundu BK, Wu HC, et al. Glucose Regulation of Load-Induced mTOR Signaling and ER Stress in Mammalian Heart. J Am Heart Assoc. 2013;2:e004796. doi: 10.1161/JAHA.113.004796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Anthony JC, Yoshizawa F, Anthony TG, Vary TC, Jefferson LS, Kimball SR. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J Nutr. 2000;130:2413–2419. doi: 10.1093/jn/130.10.2413. [DOI] [PubMed] [Google Scholar]
- 179.D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 180.Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends Mol Med. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 181.Gulati P, Gaspers LD, Dann SG, Joaquin M, Nobukuni T, Natt F, Kozma SC, Thomas AP, Thomas G. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110:163–175. doi: 10.1016/s0092-8674(02)00808-5. [DOI] [PubMed] [Google Scholar]
- 183.Koopman R, Pennings B, Zorenc AH, van Loon LJ. Protein ingestion further augments S6K1 phosphorylation in skeletal muscle following resistance type exercise in males. J Nutr. 2007;137:1880–1886. doi: 10.1093/jn/137.8.1880. [DOI] [PubMed] [Google Scholar]
- 184.Beugnet A, Tee AR, Taylor PM, Proud CG. Regulation of targets of mTOR (mammalian target of rapamycin) signalling by intracellular amino acid availability. Biochem J. 2003;372:555–566. doi: 10.1042/BJ20021266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Anthony JC, Anthony TG, Kimball SR, Vary TC, Jefferson LS. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J Nutr. 2000;130:139–145. doi: 10.1093/jn/130.2.139. [DOI] [PubMed] [Google Scholar]
- 186.Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274:11647–11652. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- 187.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Dardevet D, Sornet C, Bayle G, Prugnaud J, Pouyet C, Grizard J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J Nutr. 2002;132:95–100. doi: 10.1093/jn/132.1.95. [DOI] [PubMed] [Google Scholar]
- 189.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Haimovitz-Friedman A, Kolesnick RN, Fuks Z. Ceramide signaling in apoptosis. Br Med Bull. 1997;53:539–553. doi: 10.1093/oxfordjournals.bmb.a011629. [DOI] [PubMed] [Google Scholar]
- 191.Torre-Villalvazo I, Gonzalez F, Aguilar-Salinas CA, Tovar AR, Torres N. Dietary soy protein reduces cardiac lipid accumulation and the ceramide concentration in high-fat diet-fed rats and ob/ob mice. J Nutr. 2009;139:2237–2243. doi: 10.3945/jn.109.109769. [DOI] [PubMed] [Google Scholar]
- 192.Rennison JH, McElfresh TA, Okere IC, Vazquez EJ, Patel HV, Foster AB, Patel KK, Chen Q, Hoit BD, Tserng KY, Hassan MO, Hoppel CL, Chandler MP. High-fat diet postinfarction enhances mitochondrial function and does not exacerbate left ventricular dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1498–1506. doi: 10.1152/ajpheart.01021.2006. [DOI] [PubMed] [Google Scholar]
- 193.Ussher JR, Folmes CD, Keung W, Fillmore N, Jaswal JS, Cadete VJ, Beker DL, Lam VH, Zhang L, Lopaschuk GD. Inhibition of serine palmitoyl transferase I reduces cardiac ceramide levels and increases glycolysis rates following diet-induced insulin resistance. PLoS One. 2012;7:e37703. doi: 10.1371/journal.pone.0037703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang L, Ussher JR, Oka T, Cadete VJ, Wagg C, Lopaschuk GD. Cardiac diacylglycerol accumulation in high fat-fed mice is associated with impaired insulin-stimulated glucose oxidation. Cardiovasc Res. 2011;89:148–156. doi: 10.1093/cvr/cvq266. [DOI] [PubMed] [Google Scholar]
- 195.Listenberger LL, Han X, Lewis SE, Cases S, Farese RV, Jr., Ory DS, Schaffer JE. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc Natl Acad Sci U S A. 2003;100:3077–3082. doi: 10.1073/pnas.0630588100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Listenberger LL, Ory DS, Schaffer JE. Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J Biol Chem. 2001;276:14890–14895. doi: 10.1074/jbc.M010286200. [DOI] [PubMed] [Google Scholar]
- 197.Paumen MB, Ishida Y, Muramatsu M, Yamamoto M, Honjo T. Inhibition of carnitine palmitoyltransferase I augments sphingolipid synthesis and palmitate-induced apoptosis. J Biol Chem. 1997;272:3324–3329. doi: 10.1074/jbc.272.6.3324. [DOI] [PubMed] [Google Scholar]
- 198.de Vries JE, Vork MM, Roemen TH, de Jong YF, Cleutjens JP, van der Vusse GJ, van Bilsen M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J Lipid Res. 1997;38:1384–1394. [PubMed] [Google Scholar]
- 199.Miller TA, LeBrasseur NK, Cote GM, Trucillo MP, Pimentel DR, Ido Y, Ruderman NB, Sawyer DB. Oleate prevents palmitate-induced cytotoxic stress in cardiac myocytes. Biochem Biophys Res Commun. 2005;336:309–315. doi: 10.1016/j.bbrc.2005.08.088. [DOI] [PubMed] [Google Scholar]
- 200.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest. 2012;122:3919–3930. doi: 10.1172/JCI63888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Guo R, Zhang Y, Turdi S, Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: Role of autophagy. Biochim Biophys Acta. 2013;1832:1136–1148. doi: 10.1016/j.bbadis.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Xu X, Hua Y, Sreejayan N, Zhang Y, Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol. 2013;5:61–63. doi: 10.1093/jmcb/mjs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li ZL, Woollard JR, Ebrahimi B, Crane JA, Jordan KL, Lerman A, Wang SM, Lerman LO. Transition from obesity to metabolic syndrome is associated with altered myocardial autophagy and apoptosis. Arterioscler Thromb Vasc Biol. 2012;32:1132–1141. doi: 10.1161/ATVBAHA.111.244061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ahn J, Kim J. Nutritional status and cardiac autophagy. Diabetes Metab J. 2013;37:30–35. doi: 10.4093/dmj.2013.37.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gottlieb RA, Mentzer RM., Jr. Autophagy: an affair of the heart. Heart Fail Rev. 2012 doi: 10.1007/s10741-012-9367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nemchenko A, Chiong M, Turer A, Lavandero S, Hill JA. Autophagy as a therapeutic target in cardiovascular disease. J Mol Cell Cardiol. 2011;51:584–593. doi: 10.1016/j.yjmcc.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rotter D, Rothermel BA. Targets, trafficking, and timing of cardiac autophagy. Pharmacol Res. 2012;66:494–504. doi: 10.1016/j.phrs.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang ZV, Ferdous A, Hill JA. Cardiomyocyte autophagy: metabolic profit and loss. Heart Fail Rev. 2012 doi: 10.1007/s10741-012-9350-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. J Mol Cell Cardiol. 2008;44:968–975. doi: 10.1016/j.yjmcc.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 210.Zaha VG, Young LH. AMP-activated protein kinase regulation and biological actions in the heart. Circ Res. 2012;111:800–814. doi: 10.1161/CIRCRESAHA.111.255505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Depre C, Rider MH, Hue L. Mechanisms of control of heart glycolysis. Eur J Biochem. 1998;258:277–290. doi: 10.1046/j.1432-1327.1998.2580277.x. [DOI] [PubMed] [Google Scholar]
- 212.Collins-Nakai RL, Noseworthy D, Lopaschuk GD. Epinephrine increases ATP production in hearts by preferentially increasing glucose metabolism. Am J Physiol. 1994;267:H1862–1871. doi: 10.1152/ajpheart.1994.267.5.H1862. [DOI] [PubMed] [Google Scholar]
- 213.Katz AM. Physiology of the heart. 5th ed Wolters Kluwer Health/Lippincott Williams & Wilkins Health; Philadelphia, PA: 2010. [Google Scholar]
- 214.Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Belke DD, Larsen TS, Gibbs EM, Severson DL. Glucose metabolism in perfused mouse hearts overexpressing human GLUT-4 glucose transporter. Am J Physiol Endocrinol Metab. 2001;280:E420–427. doi: 10.1152/ajpendo.2001.280.3.E420. [DOI] [PubMed] [Google Scholar]
- 216.Schonekess BO, Brindley PG, Lopaschuk GD. Calcium regulation of glycolysis, glucose oxidation, and fatty acid oxidation in the aerobic and ischemic heart. Can J Physiol Pharmacol. 1995;73:1632–1640. doi: 10.1139/y95-725. [DOI] [PubMed] [Google Scholar]
- 217.Puthanveetil P, Wan A, Rodrigues B. FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell survival. Cardiovasc Res. 2013;97:393–403. doi: 10.1093/cvr/cvs426. [DOI] [PubMed] [Google Scholar]
- 218.Goodwin GW, Taegtmeyer H. Regulation of fatty acid oxidation of the heart by MCD and ACC during contractile stimulation. Am J Physiol. 1999;277:E772–777. doi: 10.1152/ajpendo.1999.277.4.E772. [DOI] [PubMed] [Google Scholar]
- 219.Onay-Besikci A, Altarejos JY, Lopaschuk GD. gAd-globular head domain of adiponectin increases fatty acid oxidation in newborn rabbit hearts. J Biol Chem. 2004;279:44320–44326. doi: 10.1074/jbc.M400347200. [DOI] [PubMed] [Google Scholar]
- 220.Yoon MJ, Lee GY, Chung JJ, Ahn YH, Hong SH, Kim JB. Adiponectin increases fatty acid oxidation in skeletal muscle cells by sequential activation of AMP-activated protein kinase, p38 mitogen-activated protein kinase, and peroxisome proliferator-activated receptor alpha. Diabetes. 2006;55:2562–2570. doi: 10.2337/db05-1322. [DOI] [PubMed] [Google Scholar]
- 221.Longnus SL, Wambolt RB, Barr RL, Lopaschuk GD, Allard MF. Regulation of myocardial fatty acid oxidation by substrate supply. Am J Physiol Heart Circ Physiol. 2001;281:H1561–1567. doi: 10.1152/ajpheart.2001.281.4.H1561. [DOI] [PubMed] [Google Scholar]
- 222.Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268:25836–25845. [PubMed] [Google Scholar]
- 223.Awan MM, Saggerson ED. Malonyl-CoA metabolism in cardiac myocytes and its relevance to the control of fatty acid oxidation. Biochem J. 1993;295(Pt 1):61–66. doi: 10.1042/bj2950061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Tom A, Nair KS. Assessment of branched-chain amino Acid status and potential for biomarkers. J Nutr. 2006;136:324S–330S. doi: 10.1093/jn/136.1.324S. [DOI] [PubMed] [Google Scholar]
- 225.Shimomura Y, Honda T, Shiraki M, Murakami T, Sato J, Kobayashi H, Mawatari K, Obayashi M, Harris RA. Branched-chain amino acid catabolism in exercise and liver disease. J Nutr. 2006;136:250S–253S. doi: 10.1093/jn/136.1.250S. [DOI] [PubMed] [Google Scholar]