Abstract
Oral squamous cell carcinoma (OSCC) is a common malignant tumor of the head and neck, and recurrence is an important prognostic factor in patients with OSCC. We explored the factors associated with recurrence of OSCC and analyzed the survival of patients after recurrence. Clinicopathologic and follow-up data of 275 patients with OSCC treated by surgery in the Cancer Institute and Hospital of Tianjin Medical University between 2002 and 2006 were analyzed. Recurrence factors were analyzed with Chi-square or Fisher's exact test and multivariate analysis. The prognosis of patients after recurrence was analyzed with the Kaplan-Meier method and log-rank test. The recurrence rate was 32.7%. The recurrence time ranged from 2 to 96 months, with a median of 14 months. Univariate analysis showed that T stage, degree of differentiation, pN stage, flap application, resection margin, and lymphovascular invasion were factors of recurrence (P < 0.05). Multivariate analysis showed that T stage, degree of differentiation, and pN stage were independent factors of recurrence (P < 0.001). The differences in gender, age, tumor site, region of lymph node metastasis, and perineural invasion between the recurrence and non-recurrence groups were not significant (P > 0.05). Kaplan-Meier and log-rank tests showed that the 2- and 5-year survival rates were significantly lower in the recurrence group than in non-recurrence group (67.6% vs. 88.0%, 31.8% vs. 79.9%, P < 0.001). Therefore, to improve prognosis, we recommend extended local excision, flap, radical neck dissection, and adjuvant chemoradiotherapy for patients more likely to undergo recurrence.
Keywords: Oral tumors, squamous cell carcinoma, recurrence, survival
Incidence of head and neck cancer is approximately 14/100,000, accounting for 16% to 40% of all malignancies. Oral squamous cell carcinoma (OSCC) is the most common malignant tumor of the head and neck, and its incidence has increased in recent years[1]. Surgery is the preferred treatment of OSCC. Despite great progress in chemotherapy, radiotherapy, and targeted therapy in the last three decades, the prognosis of OSCC is poor due to aggressive local invasion and metastasis, leading to recurrence. Thus, OSCC is still a challenging disease to treat in the field of head and neck cancer. Recurrence is an important prognostic factor in patients with OSCC. Camisasca et al.[2] have reported that the 5-year survival rate was 92% in OSCC patients without recurrence and 30% in patients with recurrence (P < 0.001, log-rank test). The median survival was 76.8 months in patients without recurrence and 42.5 months in patients with recurrence (P < 0.001, log-rank test). Lindenblatt et al.[3] have reported that recurrence affected the 5-year survival rate and disease-free survival of patients with OSCC. Postoperative tumor recurrence leads to a poor prognosis and a poor quality of life. Identifying factors that affect the recurrence of OSCC to reduce postoperative recurrence is an emerging issue in clinic.
In this study, we collected clinicopathologic and follow-up data of patients with OSCC and analyzed recurrence factors and patient survival. Our study provides a basis to develop standardized treatment protocols for OSCC.
Materials and Methods
Patients
All patients were treated at Tianjin Medical University Cancer Hospital between January 2002 and December 2006. Patient inclusion criteria included the following: (1) all patients were first treated, (2) all patients underwent surgery, (3) primary OSCC was confirmed by both preoperative and postoperative pathologic examination, and (4) complete clinicopathologic and follow-up data were available. Local recurrence, regional recurrence, or both were all defined as recurrence. Time to recurrence was determined by the duration from the first surgery to pathologically confirmed recurrence.
Treatment
Patients underwent surgery with other combined therapies. Patients at clinical stages I-II were treated with preoperative induction chemotherapy and surgery; patients at stages III-IV underwent preoperative chemotherapy, surgery, and postoperative radiotherapy or chemotherapy. TFP regimen was used for both preoperative and postoperative adjuvant chemotherapy: docetaxel at 60 mg/m2, day 1; cisplatin at 60 mg/m2, days 2 to 4; and 5-fluorouracil at 750 mg/m2, days 2 to 6. Simple primary tumor resection or extended tumor resection was performed according to tumor extent. For patients with poorly differentiated tumors at stages T3-T4, extended resection with a distance of at least 2 cm from the primary tumor was performed. Large wounds were repaired with skin flaps, such as the pectoralis major free flap, anterolateral thigh flap, fibular flap, and radial forearm flap. Selective neck dissection in the ipsilateral cervical I-III regions was performed for cN0 patients; radical neck dissection in the ipsilateral cervical I-V regions was performed for cN+ patients. External beam radiation was used for radiotherapy. Patients with cervical recurrence and primary recurrence underwent rescue therapies, such as primary tumor resection, neck dissection, palliative chemotherapy, and radiotherapy.
Follow-up
Patients were followed up by hospital revisits, home visits, phone calls, or mails. The final date of follow-up was December 31, 2011. All patients were followed up for at least 5 years.
Statistical analysis
SPSS16.0 was used for statistical analysis. The relationships between clinicopathologic factors for OSCC and recurrence were analyzed using Chi-square or Fisher's exact test. The Kaplan-Meier method and log-rank test were used for survival analysis. P < 0.05 was considered significant.
Results
General information
A total of 312 patients with OSCC were admitted between January 2002 and December 2006 to the Tianjin Medical University Cancer Hospital: 37 were lost to follow-up, and 275 met the inclusion criteria. The 275 patients ranged from 24 to 83 years old, with a median age of 58.5 years. The male to female ratio was approximately 1.9:1. Tongue cancer was the most common form of OSCC, followed by gingival cancer. Of the 275 cases, 214 were highly differentiated, 58 were moderately differentiated, and 3 were poorly differentiated. According to the 2002 UICC staging for OSCC, 75 cases were at stage T1, 107 at stage T2, 29 at stage T3, and 64 at stage T4; 103 had pathologically confirmed lymph node metastasis, and 172 did not (Table 1).
Table 1. Analysis of recurrence factors in 275 patients with oral squamous cell carcinoma (OSCC).
| Variable | Total (cases) | Recurrence [cases (%)] | Non-recurrence [cases (%)] | P |
| Gender | 0.633 | |||
| Male | 181 | 61 (33.7) | 120 (66.3) | |
| Female | 94 | 29 (31.9) | 65 (69.1) | |
| Tumor site | 0.966 | |||
| Tongue | 127 | 39 (30.7) | 88 (69.3) | |
| Gums | 80 | 28 (35.0) | 52 (65.0) | |
| Cheek | 31 | 11 (35.5) | 20 (64.5) | |
| Palate | 13 | 4 (30.8) | 9 (69.2) | |
| Floor of the mouth | 24 | 8 (33.3) | 16 (66.6) | |
| Age | 0.796 | |||
| ≤60 years | 168 | 54 (32.1) | 114 (67.9) | |
| >60 years | 107 | 36 (33.6) | 71 (66.4) | |
| T stage | 0.000 | |||
| T1 + T2 | 182 | 37 (20.3) | 145 (84.6) | |
| T3 + T4 | 93 | 53 (57.0) | 40 (43.0) | |
| Differentiation | 0.000 | |||
| Well | 214 | 41 (19.2) | 173 (80.8) | |
| Moderate/poor | 61 | 49 (80.3) | 12 (19.7) | |
| pN stage | 0.000 | |||
| pN0 | 172 | 40 (23.3) | 132 (76.7) | |
| pN1 + pN2 | 103 | 50 (48.5) | 53 (51.5) | |
| Skin flap application | 0.002 | |||
| Yes | 62 | 10 (16.1) | 52 (83.9) | |
| No | 213 | 80 (37.6) | 133 (62.4) | |
| Region of lymph node metastasis | 0.591 | |||
| Regions I-III | 42 | 19 (45.2) | 23 (54.8) | |
| Regions IV and V | 23 | 10 (43.5) | 13 (56.5) | |
| Regions I-V | 38 | 21 (55.3) | 17 (44.7) | |
| Resection margin | 0.001 | |||
| Negative | 221 | 62 (28.1) | 159 (71.9) | |
| Positive | 54 | 28 (51.9) | 26 (48.1) | |
| Perineural invasion | 0.630 | |||
| None | 220 | 70 (31.8) | 150 (68.2) | |
| Present | 55 | 20 (36.4) | 35 (63.6) | |
| Lymphovascular invasion | 0.006 | |||
| None | 242 | 72 (29.8) | 170 (70.2) | |
| Present | 33 | 18 (54.5) | 15 (45.5) |
All patients underwent 2 cycles of preoperative neoadjuvant chemotherapy. Forty patients underwent postoperative radiotherapy with radiation doses of≥ 60 Gy for primary tumors,≥ 60 Gy for neck regions with lymph node invasion, and≥ 50 Gy for neck regions without lymph node invasion. Sixty-five patients underwent 1 to 4 cycles of postoperative adjuvant chemotherapy. Seventy-five underwent extended resection, and 62 had flap repair. Some patients abandoned treatment because of personal reasons.
Recurrence factors
Ninety (32.7%) patients had recurrence. Recurrence time ranged from 2 to 96 months, with a median time of 14 months. The tumor recurred in the neck in 45 patients, at the primary tumor site in 36 patients, and at both the primary site and neck in 9 patients. We performed univariate analysis between various clinicopathologic factors and OSCC recurrence. A lower recurrence rate was related with T1-T2 stage, well differentiation, pN0 stage, flap repair, negative tumor resection margin, and no extracapsular invasion (P < 0.05); gender, age, primary tumor site, regions of lymph node metastasis, and perineural invasion were not related to recurrence (P > 0.05) (Table 1).
Multivariate analysis showed that T stage, degree of differentiation, pN stage, and flap repair were associated with recurrence (P < 0.001) (Table 2).
Table 2. Multivariate analysis of factors associated with recurrence of OSCC.
| Variate | B | SE | P | 95% CI for EXP(B) |
| T stage | 1.479 | 0.609 | 0.015 | 1.330-14.479 |
| pN stage | 2.408 | 0.643 | < 0.001 | 3.155-39.159 |
| Differentiation | -4.823 | 0.890 | < 0.001 | 0.001-0.046 |
SE, standard error; CI, confidence interval.
Relapse-free survival
Seventy-two patients died due to tumor-related diseases, and 90 had recurrence. The 5-year overall survival rate was 54.5%. The survival time ranged from 6 to 120 months, with a median of 36 months. The Kaplan-Meier method and log-rank test showed that the 2- and 5-year survival rates were lower in patients with recurrence than in those without recurrence (67.6% vs. 88.0%, 31.8% vs. 79.9%, P < 0.001) (Figure 1).
Figure 1. Kaplan-Meier survival curves of recurrence and non-recurrence groups.
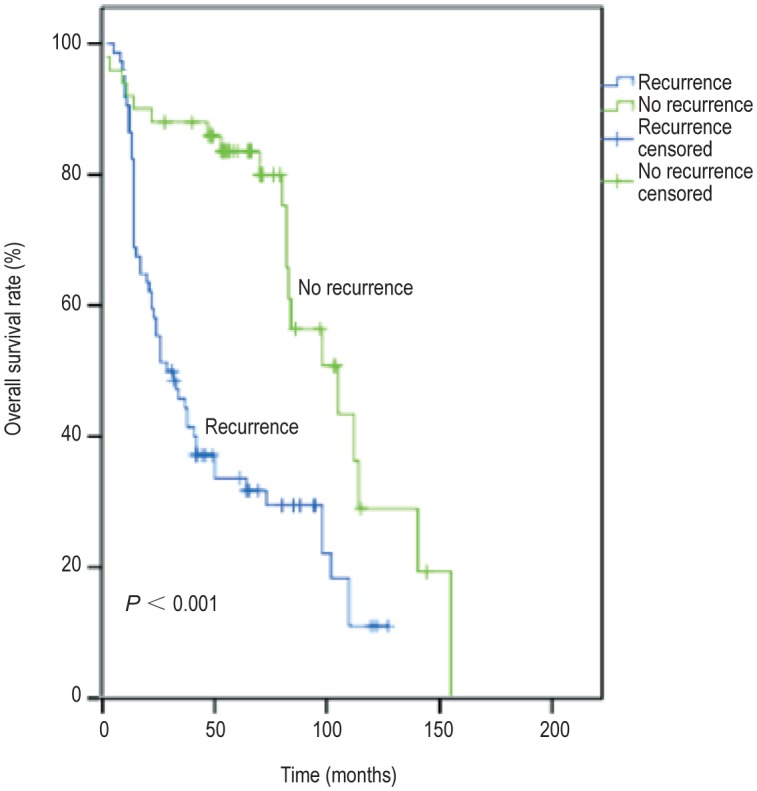
The 2- and 5-year survival rates were lower in the recurrence group than in the non-recurrence group (67.6% vs. 88.0%, 31.8% vs. 79.9%, P < 0.001).
Discussion
In this study, 90 (32.7%) patients had recurrence. Chi-square or Fisher's exact test and multivariate analysis showed that T stage, degree of differentiation, and pN stage were important factors of recurrence (P < 0.001). The 2- and 5-year survival rates were lower in patients with recurrence than in those without, as determined by the Kaplan-Meier method and log-rank test (P < 0.001).
Factors that influence the recurrence of OSCC have been extensively explored in recent years. Ebrahimi et al.[4] have reported that T stage and N stage were important factors affecting regional recurrence in OSCC. Camisasca et al.[2] have analyzed patient clinicopathologic data, including tumor sites, clinical and pathologic stage, histological grade, invasion mode, and perineural invasion. They have concluded that tongue cancer and poor differentiation contributed to OSCC recurrence after surgery. Vázquez-Mahía et al.[5] have reported that the recurrence rate was 44.9% in 118 patients with OSCC. Statistical analysis showed that co-morbidities, degree of tumor differentiation, and tumor stage were important prognostic factors for recurrence. Using univariate Chi-square or Fisher's exact test, we found that T stage, degree of tumor differentiation, pN stage, and flap repair were significantly related with tumor recurrence (P < 0.001). In our study, the recurrence rate was 32.7%, which was lower than the reported 35.5% to 47.1%[2],[4]. In the studies by Camisasca et al.[2] and Ebrahimi et al.[4], the patients underwent surgery only. Therefore, the lower recurrence rate in our study may be due to the following conditions: (1) preoperative 1-2 cycles of TPF neoadjuvant chemotherapy, (2) patients at advanced stages underwent postoperative 1-4 cycles of adjuvant chemotherapy or radiotherapy, or (3) complete tumor resection achieved with the help of various flaps. Therefore, we believe that T3-T4 stage, poor tumor differentiation, and pN positivity are important factors for the recurrence of OSCC. In addition, flap repair, adjuvant chemotherapy, or radiotherapy may also reduce recurrence.
Identifying relevant factors of tumor recurrence can help establish treatment standards. Surgery remains the preferred treatment for OSCC. However, for patients at T3-T4 stages and with poorly differentiated tumors, primary tumor resection margin should be expanded, generally 2 cm or more from the tumor, to ensure surgical safety. Flap repair should also be performed. Our results showed that the application of flap repair significantly reduced local tumor recurrence. de Vicente et al.[6] have followed up 98 patients with OSCC. They found that the mortality was 47.0% in patients with flap repair and was 67.3% in patients without flap repair (P < 0.05). Therefore, the application of free flap repair can improve the 5-year survival rate of patients. In addition, neck lymph nodes should be carefully cleaned while resecting the primary tumor. For patients with cN0 diseases, lymph nodes in the ipsilateral neck I-III regions should be selectively cleaned. Capote et al.[7] have performed selective neck lymph node dissection on pT1N0M0 patients and primary tumor resection on patients with pT2N0M0 tumors. They found that the regional recurrence rate was significantly lower in patients who underwent selective neck lymph node dissection than in those who underwent primary tumor resection only. Thus, neck lymph node dissection is an important prognostic factor for the recurrence of OSCC. For neck lymph node-positive patients, radical neck dissection should be performed in the ipsilateral carotid I-V region. Because OSCC might migrate to the IIb region, the sternocleidomastoid should be removed during surgery. Preoperative neoadjuvant chemotherapy and postoperative adjuvant chemotherapy or radiotherapy can also reduce recurrence and improve prognosis. All patients in this study underwent 1-2 cycles of preoperative neoadjuvant chemotherapy, and patients in advanced stages were treated with 4 cycles of adjuvant chemotherapy or radiotherapy after surgery. The recurrence rate was 32.7%, and the 5-year survival rate was 54.5%, both of which were satisfactory. Cooper et al.[8] have also reported that postoperative radiotherapy and chemotherapy can improve disease-free survival and improve local and regional control rate in patients with head and neck squamous cell carcinoma. López Rodríguez et al.[9] have reported that preoperative radiotherapy and chemotherapy for head and neck squamous cell carcinoma at N2-N3 stage can completely control neck lymph node metastasis and achieve local and regional effectiveness.
In this study, we explored clinicopathologic factors of recurrence in OSCC and discussed some perspectives for clinical reference. In recent years, the expression of certain genes has been reported to closely relate to the recurrence of OSCC. Cheng et al.[10] have reported that serum placental growth factor (PIGF) level could be used as a biomarker to predict the therapeutic effect on OSCC, as well as its recurrence and prognosis. Liu et al.[11] have reported that vimentin up-regulation and E-cadherin and β-catenin down-regulation were associated with recurrence and survival of OSCC patients. Therefore, our future work will explore mechanisms of OSCC recurrence at the molecular level to develop better treatment strategies.
References
- 1.Bagan JV, Scully C. Recent advances in oral oncology 2007: epidemiology, aetiopathogenesis, diagnosis and prognostication. Oral Oncol. 2008;44:103–108. doi: 10.1016/j.oraloncology.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Camisasca DR, Silami MA, Honorato J, et al. Oral squamous cell carcinoma clinicopathological features in patients with and without recurrence. ORL J Otorhinolaryngol Relat Spec. 2011;73:170–176. doi: 10.1159/000328340. [DOI] [PubMed] [Google Scholar]
- 3.Lindenblatt Rde C, Martinez GL, Silva LE, et al. Oral squamous cell carcinoma grading systems——analysis of the best survival predictor. J Oral Pathol Med. 2012;41:34–39. doi: 10.1111/j.1600-0714.2011.01068.x. [DOI] [PubMed] [Google Scholar]
- 4.Ebrahimi A, Clark JR, Zhang WJ, et al. Lymph node ratio as an independent prognostic factor in oral squamous cell carcinoma. Head Neck. 2011;33:1245–1251. doi: 10.1002/hed.21600. [DOI] [PubMed] [Google Scholar]
- 5.Vázquez-Mahía I, Seoane J, Varela-Centelles P, et al. Predictors for tumor recurrence after primary definitive surgery for oral cancer. J Oral Maxillofac Surg. 2011;70:1724–1732. doi: 10.1016/j.joms.2011.06.228. [DOI] [PubMed] [Google Scholar]
- 6.de Vicente JC, Rodríguez-Santamarta T, Rosado P, et al. Survival after free flap reconstruction in patients with advanced oral squamous cell carcinoma. J Oral Maxillofac Surg. 2012;70:453–459. doi: 10.1016/j.joms.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Capote A, Escorial V, Rodrı´guez-Campo FJ, et al. Elective neck dissection in early-stage oral squamous cell carcinoma—dose it influence recurrence and survival? Head Neck. 2007;29:3–11. doi: 10.1002/hed.20482. [DOI] [PubMed] [Google Scholar]
- 8.Cooper JS, Pajak TF, Forastiere AA, et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous cell carcinoma of the head and neck. N Engl J Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. [DOI] [PubMed] [Google Scholar]
- 9.López Rodríguez M, Cerezo Padellano L, Martín Martín M, et al. Neck dissection after radiochemotherapy in patients with locoregionally advanced head and neck cancer. Clin Transl Oncol. 2008;10:812–816. doi: 10.1007/s12094-008-0294-6. [DOI] [PubMed] [Google Scholar]
- 10.Cheng SJ, Lee JJ, Cheng SL, et al. Increased serum placenta growth factor level is significantly associated with progression, recurrence and poor prognosis of oral squamous cell carcinoma. Oral Oncol. 2012;48:424–428. doi: 10.1016/j.oraloncology.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Liu LK, Jiang XY, Zhou XX, et al. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: correlation with the clinicopathological features and patient outcome. Mod Pathol. 2010;23:213–224. doi: 10.1038/modpathol.2009.160. [DOI] [PubMed] [Google Scholar]


