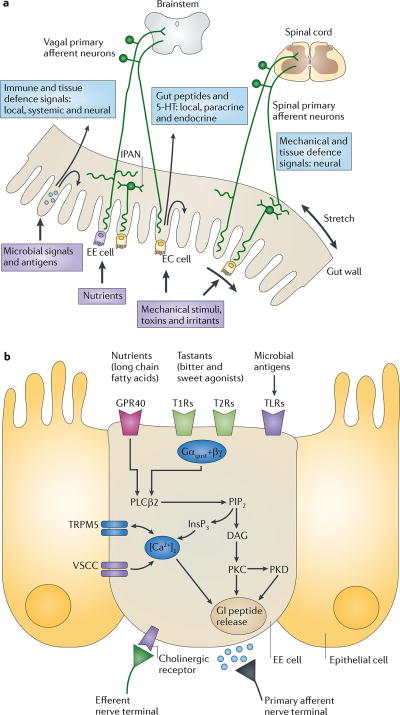
Figure 1. Gut to brain communication.
a | Endocrine, immune and neuronal afferent signalling from the gut to the CNS. Information about luminal factors and conditions of the gut are signalled through extrinsic vagal and spinal afferents to the brain stem and spinal cord, respectively. Mechanical stimuli (stretch, pressure, distortion and shearing forces) can activate spinal, vagal and intrinsic primary afferents (IPANs) directly, without intermediary cells such as the enteroendocrine (EE) cells. Although no synaptic connections have been found between IPANs and extrinsic afferents, the latter form networks around myenteric ganglia (intraganglionic laminar endings27), many of which receive synaptic input from IPANs. Signalling molecules (including proteases, histamine, serotonin and cytokines) that are produced by immune cells in Peyer's patches and within the gut epithelium can activate their respective receptors on vagal and spinal afferents. Similarly, neuropeptides and hormones (gut peptides) that are released from EE cells in response to other luminal factors, such as nutrients, toxins or antigens, can act both in an endocrine fashion, reaching targets in the brain (area postrema, dorsal vagal complex and hypothalamus), and through receptor activation on spinal and vagal afferents, in a paracrine fashion. Enterochromaffin (EC) cells signal to both IPANs and vagal afferents. b | Encoding of multiple luminal signals by EE cells. Different classes of EE cells are interspersed between gut epithelial cells throughout the gastrointestinal tract. Upon luminal stimulation (or upon activation by postganglionic sympathetic or vagal nerves), these cells can release up to 20 different gut peptides from their basolateral (and possibly luminal) surface. Released peptides can activate closely adjacent vagal afferent nerve terminals in a paracrine fashion, or when released into the circulation they can exert an endocrine effect, signalling to various sites in the brain and other parts of the gastrointestinal tract. Different types of receptors have been identified on the luminal side of EE cells, including G protein-coupled taste receptors (GPCRs) for sweet and bitter tastants, GPCRs that are responsive to fatty acids and toll-like receptors (TLRs). The intestinal taste receptors that are shown are coupled to a specific Gα protein subunit, gustducin (Gαgust), and receptor-induced increases in intracellular calcium result in peptide release from the basolateral membrane. [Ca2+]i, intracellular calcium concentration; DAG, diacylglycerol; GI peptide, gastrointestinal peptide; GPR40, G protein-coupled receptor 40; InsP3, Inositol-1,4,5-trisphosphate;. PIP2, aquaporin PIP2 member; PKC, protein kinase C; PLCβ2, phospholipase Cβ; T1R, taste receptor type 1 member; TRPM5, transient receptor potential cation channel subfamily M member 5 (specifically linked to taste receptor signalling); VSCC, voltage-sensitive Ca2+ channel.