Figure 2.
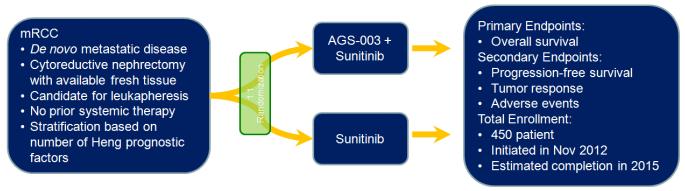
Schema showing the randomization employed in the phase III assessment of AGS-003. AGS-003 is delivered every six weeks for a total of 48 weeks. Patients with stable disease or better at 48 weeks may continue AGS-003 injections quarterly until progressive disease is noted.
