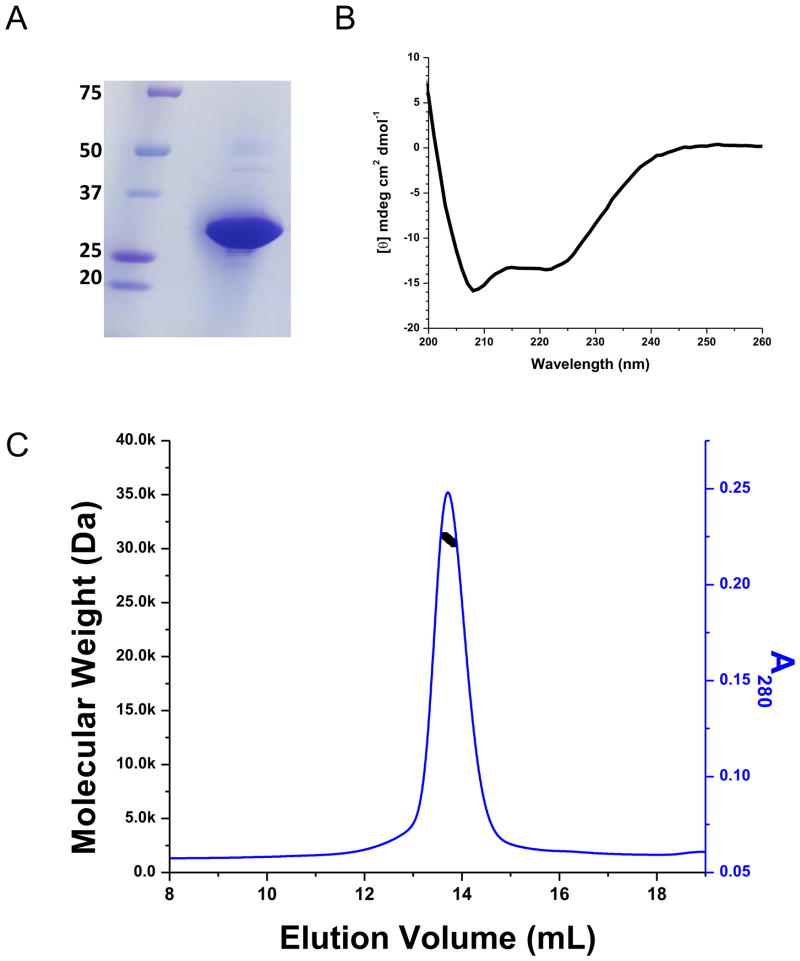
Figure 3.
Ci-VSD characterization. (A) SDS-PAGE gel of Ci-VSD-1-260. The sample was overloaded to amplify the impurities. (B) Circular Dichroism spectra in molar ellipticity of Ci-VSD in Anzergent 3-14. The calculated helicity is 52%, which is consistent with high helical contents for the expected four transmembrane helices. It suggests that Ci-VSD maintained its secondary structure in the tested detergent and the N-terminal region 1–100 is most likely unstructured. (C) Ci-VSD’s molecular weight determined by multiple angle light scattering methods in Anzergent 3-14. The molecular weight (black line) is evenly distributed throughout the peak region (blue line) indicating a homogeneous protein-detergent complex.