Abstract
Purpose: The antimicrobial activity of doripenem in comparison of imipenem, meropenem and ertapenem among Pseudomonas aeruginosa isolated from burn and Cystic Fibrosis (CF) patients were determined. Methods: Metallo-β-lactamase (MBL) genes in imipenem non susceptible P. aeruginosa isolates were detected using PCR method. The in vitro susceptibilities of doripenem, imipenem, meropenem and ertapenem were determined by Etests. MIC50 and MIC90 for corresponding antibiotics were determined individually in burn and CF isolates. Results: Among isolates which were resistant to imipenem, 16 isolates were positive for the bla IMP gene. All isolates had no bla VIM gene. All MBL producing isolates were excluded. MIC50/MIC90 of doripenem in CF and burn isolates were 0.75/>32 and >32/>32 mg/L respectively. The corresponding values for imipenem in CF and burn isolates were 2/>32 and >32/>32 mg/L, respectively. Conclusion: The susceptibility rate of doripenem is higher than that of imipenem and meropenem among P.aeruginosa isolated from CF patients, whereas, there is no difference between the efficiency of doripenem and old carbapenems in non MBL producing P.aeruginosa isolates in burn patients.
Keywords: Pseudomonas aeruginosa, Doripenem, Imipenem, Burn, Cystic fibrosis, Iran
Introduction
The synthesis of new carbapenem remain an area of intense research because of the broad-spectrum antibacterial activity of this chemical class.1-3 Doripenem is a recently released antibiotic with significant potential for use in Pseudomonas aeruginosa infections occur in CF and burn patients.4
The In vitro antimicrobial activity of Doripenem, is generally comparable to that of meropenem and imipenem although it is more active against Gram-negative organisms than imipenem.5 The activity of doripenem against P. aeruginosa isolates is slightly better than that of other carbapenems. However, development of carbapenem resistance may significantly compromise their efficacy.6 Resistance to carbapenems including doripenem resulted from the complex interaction of several mechanisms including loss of the OprD porin, overexpression of efflux systems (MexAB-OprM, MexEF-OprN) and production of carbapenemase activity, usually a metallo-β-lactamase (MBL).7-10 It should be noted that doripenem is no less susceptible to hydrolysis by MBL than are the other carbapenems and none of them is active against P. aeruginosa isolates harboring various MBL genes.11 Since, there is no CLSI guideline for doripenem MIC breakpoint until now, so the results of MIC susceptibility pattern obtained from different geographical regions from different clinical isolates could be helpful in this regard.
Since, P. aeruginosa is one of the most frequently isolated pathogens from both CF and burn patients, we designed the study to determine susceptibility patterns of all the isolates and to compare the in vitro antibacterial activity of doripenem with that of imipenem, and meropenem among non MBL P. aeruginosa isolates from both CF and burn patients.
Materials and Methods
Bacterial Strains
From June to December 2011, a total number of 92 non repetitive P. aeruginosa isolates was enrolled in this study. Sixty three burns isolates were recovered from hospitalized patients in a level one burn care center and 29 isolates were collected from CF patients admitted to a children’s medical center. This collection of bacteria was identified by conventional biochemical tests.
Antimicrobial Susceptibility Testing
The Kirby-Bauer disk diffusion method was employed to evaluate susceptibility of the following antimicrobial agents: piperacillin/tazobactam, aztreonam, ticarcillin, trimetoprime and tobramycin (MAST, UK). MIC values of the imipenem, meropenem, ertapenem (AB BIODISK, Solna, Sweden), doripenem, ceftazidime, cefepime, ciprofloxacin, amikacin, gentamicin (Liofilchem, Italy) were determined by Etests. Results were interpreted according to Clinical and Laboratory Standards Institute (CLSI) criteria, where applicable12. FDA interpretive criteria were applied to doripenem results (susceptible ≤ 2 mg/l for P. aeruginosa).13 The results were examined to ensure that reported MICs were within acceptable standards set by CLSI based on a comparator agent and the following ATCC quality control strain, ATCC 25922 (E. coli).
Ethical Standards
Ethical approval to perform the study was obtained from the institutional review board of Tabriz University of Medical Sciences. Written informed consent was obtained from all patients included in the study.
MIC50 and MIC90 Calculation
The concentration of each antimicrobial agent, that inhibited 50% (MIC 50) and 90% (MIC 90) of the strains, was calculated for each of the antibiotics singly. The formula of geometric means was used as follows:14
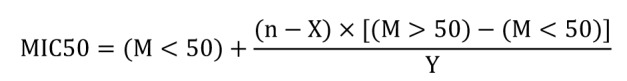
Where M < 50 is the MIC of the highest cumulative percentage below 50%, M > 50 is the MIC of the lowest cumulative percentage above 50%; n is 50% of the number of organisms tested, X is the number of organisms in the group at M <50, and Y is the number of organism in the group at M >50.
Screening for Metallo Β-Lactamase (Mbl) Production
In order to identify MBL producing isolates, we detected non susceptible isolates against imipenem by Kirby-Bauer disk diffusion method using imipenem disk 10 mg/L. Imipenem non susceptible isolates were selected for detection of IMP and VIM metalloenzymes. Total genomic DNA of the isolates which were resistant to imipenem, was extracted as described previously.15 Genes encoding class B carbapenemases were detected by PCR using specific primers for blaIMP and blaVIM metalloenzyme genes. The sequences of primers were as follows: IMP-F1, (CATGGTTTGGTGGTTCTTGT), IMP-R1, (GTAAGTTTCAAGAGTGATGC), VIM-F1, (GTTTGGTCGCATATCGCAAC) and VIM-R1 (CTACTCGGCGACTGAGCGAT). The generated PCR products were 524 and 623 base pairs, respectively.
Results
Screening MBL Production
PCR Screening of isolates which were non susceptible to imipenem indicated the presence of 17 P. aeruginosa isolates harboring bla IMP gene. Only one isolate among CF and 16 isolates among burn patients were detected as MBL positive isolates. There was no bla VIM carrying isolate detected in our study. All MBL producing isolates were excluded to remove the effect of one of the most interfering factors involved in carbapenem resistance. To the best of our knowledge, our study was the first report of Iran that evaluated the in vitro activity of doripenem in comparison with that of previously FDA approved carbapenems.
Antimicrobial Susceptibility Testing
As shown in Table 1, the Kirby-Bauer disk diffusion method was performed in 75 non MBL P. aeruginosa which comprised of 47 burn and 28 CF isolates. Table 2, summarizes the MIC’s of some antimicrobial agents other than those mentioned in Table 1. The tables showed that among the tested comparators, piperacillin/tazobactam (14.9% and 93.1% susceptible, respectively) provided the greatest activity in both burn and CF isolates, followed by tobramycin (12.8%) in burn isolates. The susceptibility rates of amikacin, imipenem, meropenem and doripenem were the same among burn isolates (10.6%). However, the susceptibility rate of doripenem among CF isolates was similar to that of amikacin (89.3%) and higher than that of old carbapenems (imipenem & meropenem). The greatest differences in the susceptibility rate between burn and CF strains were observed with doripenem (10.6% versus 89.3%), amikacin (10.6% versus 89.3%) and piperacillin/tazobactam (14.9 % versus 93.1% ).
Table 1. Results of disk diffusion method on non-MBL P. aeruginosa isolates.
| Antibiotic | PTZ | ATM | TN | TM | TC | |||||
| Profile | S n(%) | NS n(%) | S n(%) | NS n(%) | S n(%) | NS n(%) | S n(%) | NS n(%) | S n(%) | NS n(%) |
| Burn(47) | 7(14.9) | 40(85.1) | 4(8.5) | 43(91.5) | 6(12.8) | 41(87.2) | 0 | 47(100) | 4(8.5) | 43(91.5) |
| CF(28) | 26(93.1) | 2(6.9) | 9(31) | 19(69) | 22(79.3) | 6(20.7) | 2(6.9) | 26(93.1) | 23(82.8) | 5(17.2) |
| PTZ: piperacillin/tazobactam, ATM: aztronam, TN: tobramycin, TM: trimetoprime, TC: ticarcillin, S: susceptible, NS: non susceptible. | ||||||||||
Table 2. In vitro activities of doripenem and comparators against non MBL P. aeruginosa isolates in burn and CF patients.
| MIC<sub>50</sub> | MIC<sub>90</sub> | Range (mg/L) | Susceptibility (%) | Cumuative % inhibited at MIC (mg/L) | |||||||||
| =0.5 | 0.75 | 1 | 1.5 | 2 | 4 | >32 | |||||||
| Amikacin | CF | 5.5 | >256 | 1->256 | 89.3 | - | - | - | - | - | - | - | |
| Burn | 256 | 256 | 3->256 | 10.6 | - | - | - | - | - | - | - | ||
| Gentamicin | CF | 6.83 | >256 | 60.7 | - | - | - | - | - | - | - | ||
| Burn | 128 | >128 | 3->256 | 8.5 | - | - | - | - | - | - | - | ||
| Cefepime | CF | 12.25 | >256 | 2->256 | 57.1 | - | - | - | - | - | - | - | |
| Burn | 256 | 256 | 0.75->256 | 6.4 | - | - | - | - | - | - | - | ||
| Ceftazidime | CF | 2 | >256 | 0.5->256 | 75.0 | - | - | - | - | - | - | - | |
| Burn | 256 | 256 | 2->256 | 8.5 | - | - | - | - | - | - | - | ||
| Ciprofloxacin | CF | 0.19 | 6.25 | 0.047->32 | 78.6 | - | - | - | - | - | - | - | |
| Burn | >32 | >32 | 0.047->32 | 8.5 | - | - | - | - | - | - | - | ||
| Imipenem | CF | 2 | >32 | 0.75->32 | 85.7 | 0 | 7.1 | 14.3 | 39.3 | 60.7 | 85.7 | 100 | |
| Burn | >32 | >32 | 0.75->32 | 10.6 | 0 | 2.2 | 2.2 | 6.5 | 8.7 | 8.7 | 100 | ||
| Meropenem | CF | 0.75 | >32 | 0.125->32 | 85.7 | 28.6 | 50.0 | 78.6 | 82.1 | 85.7 | 89.3 | 100 | |
| Burn | >32 | >32 | 0.15->32 | 10.6 | 8.5 | 8.5 | 10.6 | 10.6 | 10.6 | 10.6 | 100 | ||
| Doripenem | CF | 0.75 | >32 | 0.094->32 | 89.3 | 39.3 | 75.0 | 82.1 | 85.7 | 85.7 | 89.3 | 100 | |
| Burn | >32 | >32 | 0.125->32 | 10.6 | 10.6 | 10.6 | 10.6 | 10.6 | 10.6 | 10.6 | 100 | ||
| Ertapenem | CF | 32 | >32 | 0.094->32 | 66.7 | 3.6 | 3.6 | 3.6 | 7.1 | 10.7 | 25.0 | 100 | |
| Burn | >32 | >32 | 3->32 | 6.4 | 0 | 0 | 0 | 0 | 0 | 8.5 | 100 | ||
According to the Table 2, among CF isolates, at any given MIC concentration from ≤0.5 to 1.5 mg/L, doripenem (MIC50, 0.75 mg/L) inhibited a slightly greater proportion of isolates than meropenem (MIC50, 0.75 mg/L) and notably greater than imipenem (MIC50, 2 mg/L ). However, higher MIC levels of doripenem at 2 and 4 mg/L, provided the same coverage as meropenem, inhibiting 85.7% and 89.3% of isolates, respectively. Table 2 showed that ertapenem was the least efficacious carbapenem (susceptibility rate, 66.7%) that could inhibit only 25% of CF isolates at the MIC level of 4 mg/L.
On the other hand, among burn isolates, all carbapenems except ertapenem had the same activity (MIC50 and MIC90, >32 mg/L). The proportion of isolates inhibited at MIC level ≥1 mg/L of doripenem and meropenem was similar (10.6%), however, the inhibition rates for doripenem at MIC levels of ≤0.5 and 0.75 mg/L were slightly higher than that of meropenem. At any given concentration from ≤ 0.5 to > 32 mg/L, doripenem inhibited a remarkably greater proportion of isolates than imipenem (Table 2). Similar to CF isolates, ertapenem identified as the least potent agent in burn isolates which inhibited 6.4% of burn isolates in comparison with 10.6% inhibition by other carbapenems.
According to the susceptibility rates, the MIC levels of imipenem, meropenem and doripenem were completely in line with each other except for 3 isolates; one burn and 2 CF isolates which showed the MIC level of imipenem of >32 mg/L but doripenem and meropenem MIC levels of ≤ 1 mg/L.
Discussion
Infections caused by P. aeruginosa in burn and CF patients often treated with difficulty due to the emergence of resistance and lack of effective antibiotics.16 Doripenem as a new carbapenem offers potentially enhanced carbapenem activity but does not expand the spectrum of activity of this class4. Like other carbapenems, doripenem has stability against many β-lactamases, but remains labile to class B enzymes, known as metallo-β-lactamases.5 Therefore, in the present work, we attempted to assess the in vitro activity of doripenem among non MBL P. aeruginosa isolated from CF and burn patients, in comparison with other carbapenems.
Despite the higher carbapenem MIC rates in our CF isolates as compared with similar studies,15,16 it can be concluded that doripenem has much greater potency than imipenem. Although the MIC50 of both doripenem and meropenem was similar, the more inhibition rate of 25% of this recently approved carbapenem indicated that doripenem could be considered as a good alternative therapeutic agent in CF patients. In a recent similar study,16 antibiotic susceptibility of P. aeruginosa isolated from CF patients, doripenem showed as the most active antibiotic in the absence of piperacillin/tazobactam. Totally, it seems that doripenem can be considered as the most potent carbapenems against P. aeruginosa infections in CF patients.
Since, MBLs were the most important mechanisms in high level of resistance against all carbapenems, we decided to exclude the MBL positive isolates to explore the probable difference in doripenem MIC’s versus old carbapenems. Although the susceptibility rates against doripenem in burn isolates showed no superiority to old carbapenems, the greater population of isolates were inhibited at any concentration of doripenem as compared with imipenem and meropenem. Among burn isolates, all cabapenems have the same activity except for ertapenem which has the least efficiency.
We found 3 imipenem resistant isolates which were susceptible to meropenem and doripenem. This phenomenon occurred to those isolates with nonenzymatic resistance involving loss of porin OprD and up-regulation of efflux pumps13 which we intend to explore in a further study. Conversely, other researchers declared that this could be the exception other than a rule with no reason.17
Although ertapenem is not a representative of carbapenems with the consideration of broad spectrum activity which can not be used to treat infections due to non-fermentative Gram-negative bacteria,13 we intended to investigate the in vitro activity of this antimicrobial agent for the first time in Iran. Our results are consistent with the results of other investigators18 which showed the lowest susceptibility rate among all used carbapenems in both burn and CF isolates (6.4% and 66.7%). Our results corroborated by the results of the study conducted by Quale et al. They found only 18% of P. aeruginosa isolates that were susceptible against ertapenem while imipenem and meropenem were more potent, inhibiting 55% and 64% of isolates compared to ertapenem.
Conclusion
Although doripenem is more active than imipenem and meropenem against P. aeruginosa isolated from CF patients, no superiority of doripenem is observed to old carbapenems in non MBL producer P. aeruginosa isolates in burn patients. In terms of MIC level of doripenem, this antibiotic is the most active but this advantage is partly offset by lower regulatory breakpoints. Ertapenem is the least potent agent against P. aeruginosa isolates.
Acknowledgments
We are grateful to Dr. Saber Yousefi from Department of Clinical Laboratory Medicine, Faculty of Paramedicine, Urmia University of Medical Sciences.
This work was supported fully by Tabriz Research Center of Infectious and Tropical Diseases (grant No.89/16), Tabriz University of Medical Sciences, Tabriz, Iran.
This is a report of a database from thesis entitled “Evaluation of MexAB-OprM and MexXY-OprM efflux systems, AmpC cephalosporinase and OprD protein expressions to investigate their association with resistance against carbapenemes in Pseudomonas aeruginosa isolated from clinical specimens” registered in Tabriz University of Medical Sciences.
Conflict of interest
There is no conflict of interest in this study.
References
- 1.Nomura S, Nagayama A. In vitro antibacterial activity of s-4661, a new parenteral carbapenem, against urological pathogens isolated from patients with complicated urinary tract infections. J Chemother. 2002;14(2):155–60. doi: 10.1179/joc.2002.14.2.155. [DOI] [PubMed] [Google Scholar]
- 2.Ohba F, Nakamura-Kamijo M, Watanabe N, Katsu K. In vitro and in vivo antibacterial activities of er-35786, a new antipseudomonal carbapenem. Antimicrob Agents Chemother. 1997;41(2):298–307. doi: 10.1128/aac.41.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe A, Takahashi H, Kikuchi T, Kobayashi T, Gomi K, Fujimura S. et al. Comparative in vitro activity of s-4661, a new parenteral carbapenem, and other antimicrobial agents against respiratory pathogens. Chemotherapy. 2000;46(3):184–7. doi: 10.1159/000007276. [DOI] [PubMed] [Google Scholar]
- 4.Parkins MD, Elborn JS. Newer antibacterial agents and their potential role in cystic fibrosis pulmonary exacerbation management. J Antimicrob Chemother. 2010;65(9):1853–61. doi: 10.1093/jac/dkq245. [DOI] [PubMed] [Google Scholar]
- 5.Lascols C, Legrand P, Merens A, Leclercq R, Armand-Lefevre L, Drugeon HB. et al. In vitro antibacterial activity of doripenem against clinical isolates from french teaching hospitals: Proposition of zone diameter breakpoints. Eur J Clin Microbiol Infect Dis. 2011;30(4):475–82. doi: 10.1007/s10096-010-1117-6. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Martinez JM, Poirel L, Nordmann P. Molecular epidemiology and mechanisms of carbapenem resistance in pseudomonas aeruginosa. Antimicrob Agents Chemother. 2009;53(11):4783–8. doi: 10.1128/AAC.00574-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Amin N, Giske CG, Jalal S, Keijser B, Kronvall G, Wretlind B. Carbapenem resistance mechanisms in pseudomonas aeruginosa: Alterations of porin oprd and efflux proteins do not fully explain resistance patterns observed in clinical isolates. Acta Pathol Microbiol Immunol Scand. 2005;113(3):187–96. doi: 10.1111/j.1600-0463.2005.apm1130306.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolter DJ, Smith-Moland E, Goering RV, Hanson ND, Lister PD. Multidrug resistance associated with mexxy expression in clinical isolates of pseudomonas aeruginosa from a texas hospital. Diagn Microbiol Infect Dis. 2004;50(1):43–50. doi: 10.1016/j.diagmicrobio.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Pai H, Kim J, Lee JH, Choe KW, Gotoh N. Carbapenem resistance mechanisms in pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2001;45(2):480–4. doi: 10.1128/AAC.45.2.480-484.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livermore DM. Of pseudomonas, porins, pumps and carbapenems. J Antimicrob Chemother. 2001;47(3):247–50. doi: 10.1093/jac/47.3.247. [DOI] [PubMed] [Google Scholar]
- 11.Rice LB. Challenges in identifying new antimicrobial agents effective for treating infections with acinetobacter baumannii and pseudomonas aeruginosa. Clin Infect Dis. 2006;43 (Suppl 2):S100–5. doi: 10.1086/504487. [DOI] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; 19th informational supplement. CLSI document M100-S19. Wayne PA: CLSI; 2009.
- 13.Castanheira M, Jones RN, Livermore DM. Antimicrobial activities of doripenem and other carbapenems against pseudomonas aeruginosa, other nonfermentative bacilli, and aeromonas spp. Diagn Microbiol Infect Dis. 2009;63(4):426–33. doi: 10.1016/j.diagmicrobio.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 14.Smith JA, Henry D, Ngui-Yen J, Castell A, Coderre S. Comparison of agar dilution, microdilution, and disk elution methods for measuring the synergy of cefotaxime and its metabolite against anaerobes. J Clin Microbiol. 1986;23(6):1104–8. doi: 10.1128/jcm.23.6.1104-1108.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bou G, Cervero G, Dominguez MA, Quereda C, Martinez-Beltran J. Pcr-based DNA fingerprinting (rep-pcr, ap-pcr) and pulsed-field gel electrophoresis characterization of a nosocomial outbreak caused by imipenem- and meropenem-resistant acinetobacter baumannii. Clin Microbiol Infect. 2000;6(12):635–43. doi: 10.1046/j.1469-0691.2000.00181.x. [DOI] [PubMed] [Google Scholar]
- 16.Traczewski MM, Brown SD. In vitro activity of doripenem against pseudomonas aeruginosa and burkholderia cepacia isolates from both cystic fibrosis and non-cystic fibrosis patients. Antimicrob Agents Chemother. 2006;50(2):819–21. doi: 10.1128/AAC.50.2.819-821.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paterson DL, Depestel DD. Doripenem. Clin Infect Dis. 2009;49(2):291–8. doi: 10.1086/600036. [DOI] [PubMed] [Google Scholar]
- 18.Quale J, Bratu S, Gupta J, Landman D. Interplay of efflux system, ampc, and oprd expression in carbapenem resistance of pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2006;50(5):1633–41. doi: 10.1128/AAC.50.5.1633-1641.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


