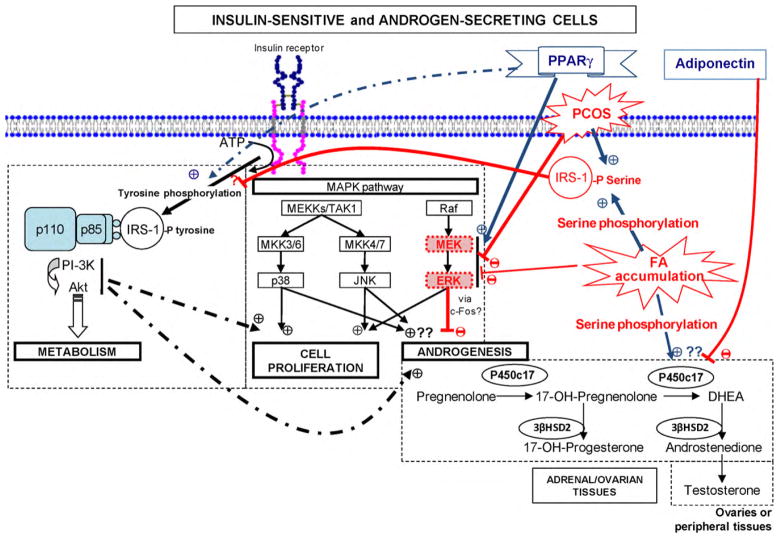
Fig. 3.
Proposed cellular mechanisms involved in insulin-stimulated androgen biosynthesis, PCOS-associated defects, free fatty acids-induced insulin resistance and increased androgen production, and PPARγ actions. Insulin binds to its receptor resulting in tyrosine phosphorylation of the receptor and insulin receptor substrates (IRSs) such as the IRS-1. IRS-1 activates phosphoinositide-3-kinase (PI-3K) and Akt, which mediate insulin-stimulated glucose metabolism. Serine phosphorylation of IRS-1 prevents its binding with PI-3K and inhibits insulin signalling. Furthermore, serine phosphorylation of P450c17 increases its 17,20-lyase activity and thus androgen biosynthesis. Interestingly, serine phosphorylation of IRS-1 is constitutively increased in PCOS women and increased by fatty acids (FAs) accumulation, and PPARγ agonists increase tyrosine phosphorylation of IRS-1. On the other hand, insulin-stimulated androgen production has been shown to be reduced by specific inhibition of PI-3K and increased by specific inhibition of MEK. MEK/ERK activity was found to be constitutively reduced in PCOS women, activated by PPARγ agonists and inhibited by FFAs. It was also suggested that P450c17 activity may be stimulated by other players of the MAPK pathway, such as MKK3/6-p38 and MKK4/7-JNK, which are at least normally functional in women with PCOS, adapted from Baillargeon [92].