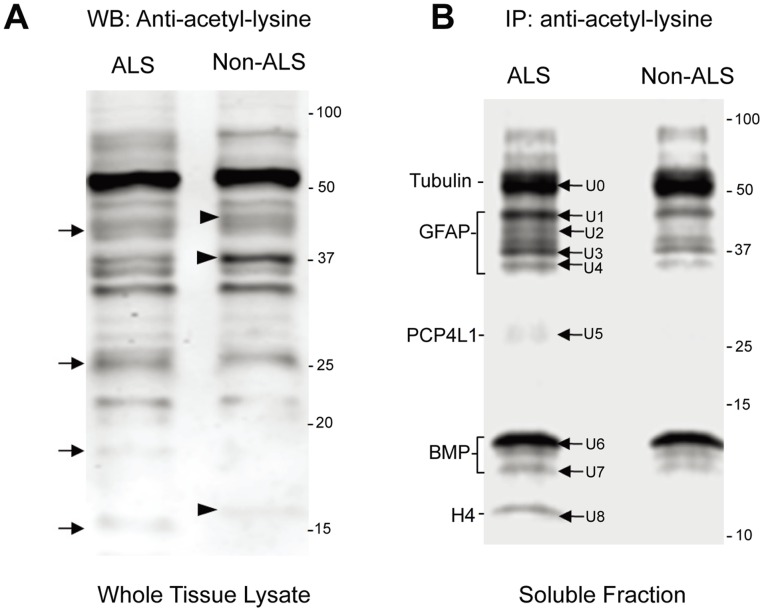
Figure 6. Differentially regulated protein acetylation in ALS and non-ALS spinal cords by Western blotting and immunoprecipitation.
(A) Western blotting analysis of total acetylated proteins. The urea-soluble proteins from ALS and non-ALS spinal cords were resolved on SDS-PAGE and followed by Western blotting using the antibody against acetyl-lysine. Arrows indicate bands found in ALS spinal cords, while arrowheads indicate the bands found in non-ALS counterparts. (B) Immunoprecipitation of the acetylated proteins. The soluble protein fractions were immunoprecipitated with the antibody against acetyl-lysine, resolved by SDS-PAGE and stained with Sypro Ruby. The protein bands labelled with U0, U1, … U8 were recovered, digested with trypsin and identified with LC-MS/MS. The proteins that were identified by LC-MS/MS are indicated to the left.