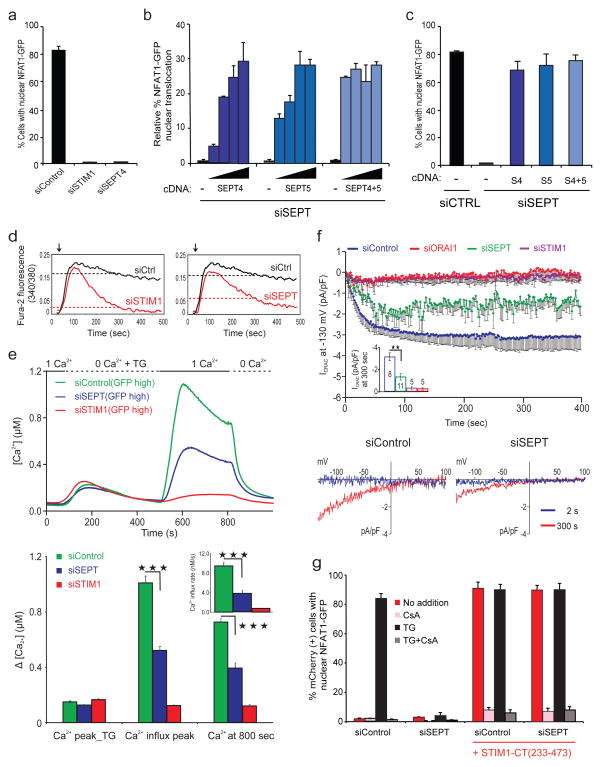
Figure 1. Mammalian septin proteins are essential regulators of NFAT activation and store-operated Ca2+ influx.
a, HeLa NFAT1-GFP cells were transfected with siRNAs, stimulated with thapsigargin (TG) and scored for nuclear NFAT1 by fluorescence imaging and automated analysis. b, siRNA-treated HeLa NFAT1-GFP cells were transfected with siRNA-resistant SEPT4 and SEPT5 cDNAs (5, 10, 15, 20 ng), stimulated with TG and scored for nuclear NFAT1. The (−) cDNA samples received empty vector (20 ng). c, Reanalysis of b (20 ng) after gating on septin-expressing cells. d, Averaged [Ca2+]i readings in siRNA-treated, fura-2 loaded HeLa NFAT1-GFP cells stimulated with TG (arrow) in 10 mM [Ca2+]o. The same siControl trace is shown in both panels. e, (Upper) Single-cell [Ca2+]i measurements in siRNA-treated, fura-2 loaded Jurkat T cells expressing high levels of co-transfected GFP (siControl, n=135; siSTIM1, n=212; siSEPT, n=120) exposed to 0 or 1 mM [Ca2+]o before and after TG stimulation. (Lower) [Ca2+]I at the peak of TG-stimulated release from ER stores in GFP-high cells; at the peak following Ca2+ add-back (siSEPT vs siControl, p=5.3×10−22); and at 800 sec (siSEPT vs siControl, p=1.6×10−16); inset, initial rates of [Ca2+]i increase (siSEPT vs siControl, p=1.4×10−13). Triple asterisks indicate statistically significant differences. f, (Upper) CRAC current density at −130 mV in Jurkat T cells; inset, CRAC current density at 300 sec (siSEPT vs siControl, p=0.002). (Lower) Current–voltage relationship recorded 2 sec and 300 sec after break-in (whole cell configuration) g, mCherry or mCherry-STIM1-CT(233–473) plasmids were expressed in siRNA-treated HeLa NFAT1-GFP cells, and mCherry-positive cells were stimulated and scored for nuclear NFAT1. Error bars report sample standard deviation (s.d.).