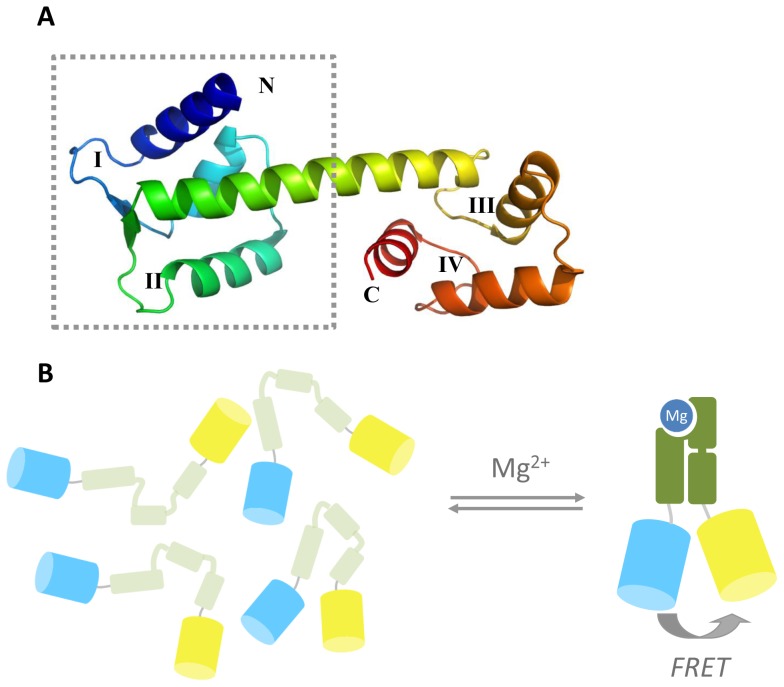
Figure 1. Design of the genetically encoded magnesium FRET sensor MagFRET.
(A) Crystal structure (PDB code 2GGM) of HsCen2 in the calcium-bound, compact state. The typical helix-loop-helix structure can be observed, with EF-hands indicated by Roman numerals. The dotted lines indicate the N-terminal truncated part of the domain used in the sensor. In HsCen3, the high-affinity Mg2+/Ca2+ binding site is in loop I, and a much weaker Ca2+-binding site is found in loop II. (B) Schematic representation of MagFRET, where the N-terminal truncation of HsCen3 is flanked by Cerulean and Citrine. In absence of Mg2+, the HsCen3 domain is in a molten globule-like state, with little tertiary structure and a relatively large average distance between the fluorescent domains. Mg2+-binding induces a compact, well-defined tertiary structure, resulting in increased energy transfer between Cerulean and Citrine.