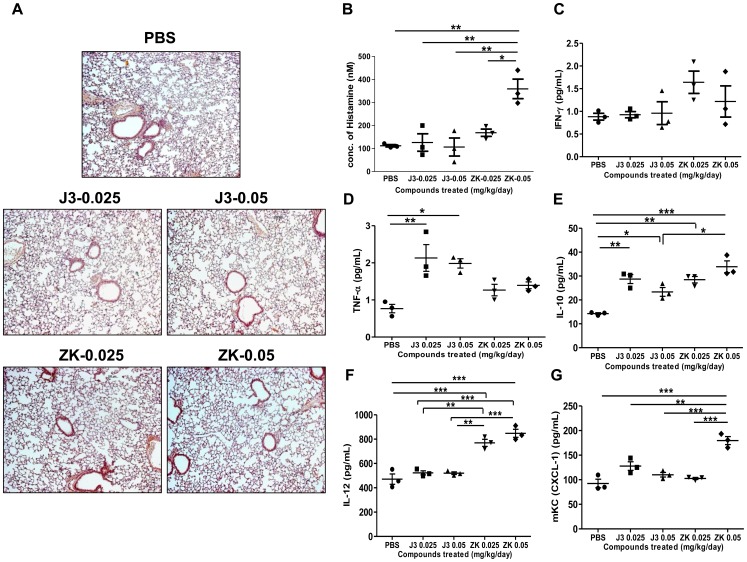
Figure 4. In vivo evaluation for hypersensitivity by chronic toxicity test.
(A) Δ12-PGJ3 (0.025 and 0.05 mg/kg body weight) and ZK118182 (0.025 and 0.05 mg/kg body weight) were injected intraperitoneally into C57BL/6 mice. Post two weeks of treatment, the alveolar tissue was extracted, fixed, and stained with H&E. Mice treated with PBS were used as placebo control. All treatments were carried out in triplicates and a representative image has been shown (magnification: 10X). (B) Histamine release assay was carried out on the plasma from the mice treated as mentioned above by EIA method. (C–G) Multi-array Th1/Th2 cytokine analysis was carried-out using the plasma, upon treatment of mice as mentioned above. The figures shown are for: (C) IFN-γ, (D) TNF-α, (E) IL-10, (F) IL-12 and (G) mKC (CXCL-1). The data shown are mean ± SEM (n = 3) and statistical significance are represented as *- p≤0.05; **- p≤0.01; ***- p≤0.001 respectively.