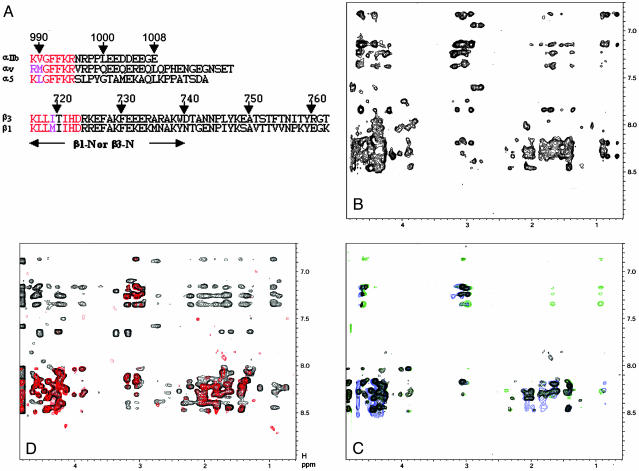
Fig. 1.
Association and dissociation of integrin αv/β3 and α5/β1 tails. (A) Primary sequences of the αIIb, αv, α5, β1, and β3 tails. Highly conserved residues involved in the membrane-proximal interface are indicated by red (identical) and pink (similar). The lengths of β1-N and β3-N with C-terminal truncations are indicated by the arrow. (B) The 2D NOESY spectrum of α5 tail in the presence of MBP-β1(α5:MBP-β1 = 1 mM:0.1 mM), showing substantial transferred NOEs due to α5/β1 tail association. (C) The 2D NOESY spectra of 1 mM α5 tail in free form (black) and in the presence of 0.1 mM MBP-β1/0.2 mM talin-H (blue) or 0.1 mM MBP-β1/0.2 mM talin F2-F3 (green). Clearly, talin-H or talin F2-F3 abolished the transferred NOEs. (D) Mathematical addition of 2D NOESY spectra of 1 mM α5 and β1-N (red, random-coiled pattern) vs. 2D NOESY spectrum of α5/β1-N mixture in 1 mM:1 mM ratio (black, highly structured with substantial number of NOEs). Note that no fusions were attached to these peptides. Mixing time is 400 ms for all experiments, which were performed at 25°C in 20 mM phosphate buffer, 5 mM Ca2+/pH 6.3.