Figure 10. Diagram showing the contribution of 2-arachidonoyl glycerol to phospholipase C-dependent activation of TRPV1.
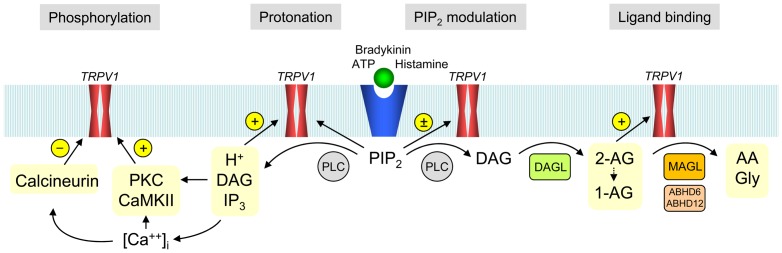
Ligand-Gq-receptor interaction causes activation of phospholipase C (PLC), hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) and formation of diacylglycerol (DAG), which is further metabolized to 2-arachidonoylglycerol (2-AG) by diacylglycerol lipase (DAGL). 2-Arachidonoylglycerol undergoes a spontaneous acylmigration to yield 1-arachidonoylglycerol (1-AG). Both 2-AG and 1-AG directly activate TRPV1, and their effects are terminated by monoacylglycerol lipase (MAGL) and related enzymes, including the alpha/beta-hydrolases ABHD6 and ABDH12. Diacylglycerol indirectly regulates TRPV1 via protein kinase C (PKC)-dependent phosphorylation of the ion channel, whereas PIP2 has complex and opposing effects on TRPV1 channel gating. Hydrolysis of PIP2 also yields inositol 1,4,5-triphosphate (IP3), which via release of intracellular calcium causes activation of calcium-calmodulin-dependent protein kinase II (CaMK II). The phosphatase calcineurin dephosphorylates TRPV1. Protein phosphorylation, protonation, PIP2 modulation and binding of membrane-derived lipids provide an intricate system for fine-tuning of TRPV1 activity.
