Abstract
Introduction:
The present study was designed to evaluate the effect of granulocyte-colony stimulating factor (G-CSF) in the treatment of Parkinson's disease (PD), the second most common neurodegenerative disease characterized by muscle and movement disorder, often associated with depression. PD is very difficult to treat. Hence, the present study was aimed to evaluate the effect of G-CSF in PD associated with depression.
Materials and Methods:
Adult Wistar male rats weighing about 180-250 g were selected and divided into five groups in parallel designed method namely; control group (n = 5); sham operated group (n = 5); Vehicle group (n = 5); G-CSF group (70 μg/kg, s.c.) (n = 5) and L-DOPA group (n = 5). The rats were treated with 6-hydroxydopamine (6-OHDA) on day 0 and then treatment was continued for 14 day of L-DOPA/carbidopa, whereas G-CSF (70 μg/kg, s.c.) was given from day 1 to 6. Thereafter, adhesive removal and forced swim tests were conducted to evaluate the behavioral outcome of G-CSF treatment. The finding was correlated and analyzed with Nissl staining findings for the final conclusion.
Results:
The behavioral parameters were assessed and found to be ameliorate the symptoms of Parkinson's and reduced the depression like behavior in PD. The histological findings were supported the behavioral findings and showed pathological improvement.
Conclusion:
As a preliminary work, the present study first time suggested that G-CSF have a potential role in PD and associated depression.
KEY WORDS: 6-hydroxydopamine, depression, forced swim test, granulocyte-colony stimulating factor, Parkinson's disease
Introduction
Parkinson's disease (PD) is one of the most common neurodegenerative movement disorders. It affects more than 0.1% of the population older than 40 years of age.[1] Clinically, most patients present with a tremor at rest, rigidity and akinesia. A number of patients also suffer from anxiety, depression, autonomic disturbances and dementia. Although there are effective symptomatic therapies, there are no proven neuroprotective or neurorestorative therapies.[2] The major clinical symptoms of PD is loss of dopamine (DA) neurons in the substantia nigra pars compacta (SNc), but there is wide-spread neuropathology and the SNc only becomes involved toward the middle stages of the disease.[3] Lewy bodies and dystrophic neurites (Lewy neurites) are a pathologic hallmark of PD and classically are round eosinophilic inclusions composed of a halo of radiating fibrils and a less defined core.[4] However, the other disorder like depression is the main confounding factor that impacts quality-of-life in PD[5,6,7] and prevalence of depression in PD varies from 20% to 50% and is frequently associated with greater disability, rapid progression of motor symptoms and increased mortality.[8,9]
Granulocyte-colony stimulating factor (G-CSF), a hematopoietic growth factor has a potential role in mobilizing peripheral blood progenitor cells and form new myeloid cells with potential neuroprotective effect in several studies.[10,11,12] Experimental evidence have shown that neuroprotective effect of G-CSF is due to inhibitory activity of the production or activity of the main inflammatory mediators interleukin-1, tumor necrosis factor-alpha and interferon gamma.[13]
Hence, present study was designed to evaluate the effect of G-CSF on experimentally induced PD and co-morbid depression in rats because it is very difficult to target multiple contributing factors by a drug.
Materials and Methods
Adult Wistar male rats weighing about 180-250 g were obtained from the central animal house of the institute. The animals were housed in standard laboratory conditions in groups of three at 25 ± 2°C, humidity of 60 ± 2% and 12 h light: Dark cycle. Animals had a free access to standard laboratory chow diet and tap water. The animals were acclimatized to the laboratory conditions 1 week prior to experimentation. All experiments were conducted daily between 09:00 am and 03.00 pm h. The protocol was approved from Institutional Animal Ethics Committee and the animal housing, care and handling was followed as per committee for the purpose of control and supervision on experiments on animals guidelines for animal care and use.
The experiment was designed as a parallel group study. The experiment was carried out in eight groups namely, control group (n = 5): In this group, healthy normal rats were taken and the vehicle (phosphate buffered saline, pH 7.4 with 0.01% ascorbic acid) was given from 1 to 14 days intraperitoneally; sham operated group (n = 5): Rats were exposed to stereotaxis/intracerebroventricular injection, but were not treated with any drug preparation, then sutured back and was analyzed on day 15. Vehicle group (n = 5): Rats were exposed to stereotaxis/intracerebroventricular injection on day 0 in the fixed coordinates and 6-hydroxydopamine (6-OHDA) at the concentration of 12 μg in 4 μl in 0.01% ascorbic acid was inserted and treatment with the vehicle for 14 days; G-CSF group (70 μg/kg, s.c.) (n = 5): Rats were exposed to stereotaxis/intracerebroventricular injection on day 0 in the fixed coordinates and 6-OHDA at the concentration of 12 μg in 4 μl in 0.01% ascorbic acid was inserted and G-CSF (70 μg/kg, s.c.) treatment was given for 1-6 days and followed for 14 days. L-DOPA group (30 mg/kg) (n = 5): Rats were exposed to stereotaxis/intracerebroventricular injection on day 0 in the fixed coordinates and 6-OHDA at the concentration of 12 μg in 4 μl in 0.01% of ascorbic acid was introduced and treated with L-DOPA/carbidopa (30 mg/7.5 mg; 4:1) for 1-14 days.
PD Model Development (6-OHDA Nigrostriatal Lesions)
Unilateral 6-OHDA (base, Sigma-Aldrich Ltd. Poole, UK) nigrostriatal lesions were created after 1 h of drug administration. Rats were anesthetized with ketamine (100 mg/kg, i.p.) + xylazine (10 mg/kg, i.p.) and placed in a stereotaxic frame. The coordinates of the substantia nigra was located at anterior-posterior (AP): –3.0 mm and middle lateral (ML): +2.5 mm (relative to bregma), dorsal ventral (DV): –7.6 mm (relative to dura).[14] Next, 4 μl (12 μg) of 6-OHDA–hydrochloride in 0.1% of ascorbic acid/saline solution was drawn into a 10 μl Hamilton syringe and infused into the substantia nigra at a rate of 1 μL/min followed by a 5-min equilibration time. Infusions were made during which the needle remained in place and was then slowly retracted.[15] After surgery, the wound of rats were wiped with 70% of ethanol and iodine solution. Then, treated with neosporin power before sifting to their home cage and were treated with parenteral analgesics daily for 5 days.
Adhesive Removal
Small adhesive stimuli (10 mm round) were placed on the snout of the rat and the time to make contact and remove the stimulus was recorded. To remove the stimulus, animals would raise both forelimbs toward their face and swipe off the stimulus with both forepaws. In general, rats make contact and remove the stimulus within 10 s. Each animal received two trials and the trials were alternated and hencethat each rat has an intertribal interval of at least 2 min. All the testing was performed in the animal's home cage and cage mates were temporarily removed during testing because they can interfere with stimulus removal. If the animals not able to remove the stimulus within 60 s, the experimenter removed it. Stimulus contact time, removal time and removal contact time was calculated for each animal.[16]
Forced swim test (FST)
FST is very commonly used model for screening and development of antidepressant drugs. It developed despair like behavior in rodents. The test The FST described by Porsolt et al.[17] 1977 was slightly modified and followed briefly, each rat was placed individually in a glass cylinder (diameter 22.5 cm, height 30 cm) that was filled to the 25 cm mark with water. The rat was forced to swim for 15 min on the 1st day of experiment (pre-test day). Rats were then allowed to return to their home cage. On day 1, 7 and 15, each rat (vehicle/drug treated) was assessed for 5 min. The duration of immobility, floating and climbing were recorded in last 4 min. Rat was immobile, when its front and hind paws were no longer moving and the rat was in floating position without struggle, whereas climbing was defined when rats were tried to move its front paws on the cylinder wall and try to climb. The rat was considered to be immobile when it stopped struggling and passively moved to remain floating and keep its head above water. Water was changed between the experiment to make rats clear and visible and the temperature was maintained at 22 ± 2°C throughout the experiment. The immobility time was recorded manually by an observer who was blind to the drug treatment.
Histological evaluation
Analysis of cell density in SNc was performed on by using Nissl Staining. Briefly, Nissl-stained neurons were analyzed in (light microscopy; ×400). At least two sections representative of each of four Paxinos–Watson planes (4.2, 3.8, 3.2, 2.97; interaural) were examined by scanning the mounted sides.[18]
Statistical Analysis
Observations were recorded and arranged on a Microsoft Excel spreadsheet (Microsoft, Seattle, WA, USA). The data was imported into statistical package of social science (SPSS version 16.0; IBM Corp. Armonk, NY) and behavioral measurements were analyzed using the one-way ANOVA post-hoc Tukey's test. The P < 0.05 was considered to be statistically significant.
Results
The surgical procedure was conducted after proper anesthesia and all animals tolerated well the surgical operations and found there was no mortality due to treatments. However, we observed there is weight loss in some rodents, but found not significantly different from one another (data not shown).
Effect Of G-CSF on Adhesive Removal Test
On day 1, reaction time to remove the adhesive from their snout was very less, however, on day 7 and 14 reaction time was significantly increased in vehicle treated group when compared to control and sham operated group (P < 0.001; 15.8 ± 2.26 vs. 2.0 ± 0.32 vs. 1.6 ± 0.24). Treatment with G-CSF 70 was found to significantly reduced the reaction time as compared to vehicle treated as well as standard drug L-DOPA (P < 0.05; 4.8 ± 1.07 vs. 24.8 ± 4.18 vs. 12.4 ± 1.50) [Figure 1].
Figure 1.
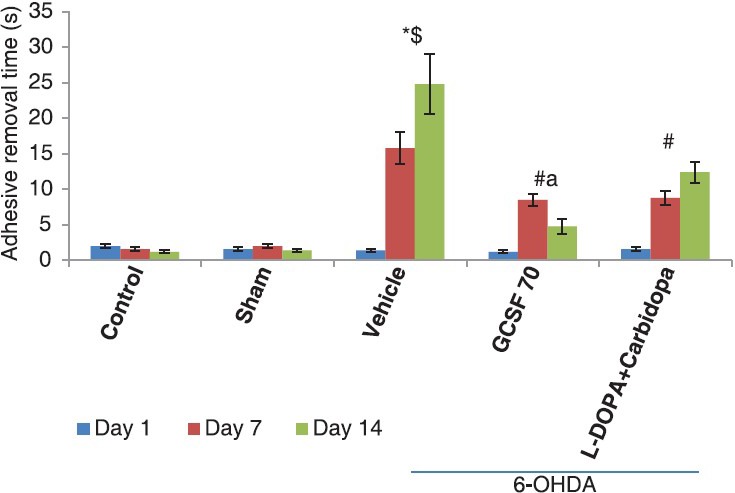
Adhesive removal test, effect of granulocyte-colony stimulating factor on adhesive removal test in 6-hydroxydopamine (6-OHDA) induced Parkinson's disease model of rats: *P< 0.05, as compared to control; $P< 0.05, as compared to sham; #P< 0.05, as compared to 6-OHDA + vehicle group, aP< 0.05, as compared to L-DOPA/carbidopa
Effect Of G-CSF on Immobility Test in Forced Swim Test
Rats were exposed to the FST on day 13 of experiment and immobility behavior was assessed on day 14. In the vehicle group, immobility time was significantly increased when compared with control and sham operated group (P < 0.001; 76.6 ± 5.56 s vs. 11.8 ± 2.05 vs. 13.8 ± 1.65) Treatment with G-CSF 70 was significantly reduced the immobility time on day 14 when compared with vehicle treated group (P < 0.005; 17.0 ± 3.03 s vs. 76.6 ± 5.56 s) and L-DOPA + carbidopa group (P < 0.05; 17.0 ± 3.03 s vs. 39.4 ± 1.72) [Figure 2].
Figure 2.
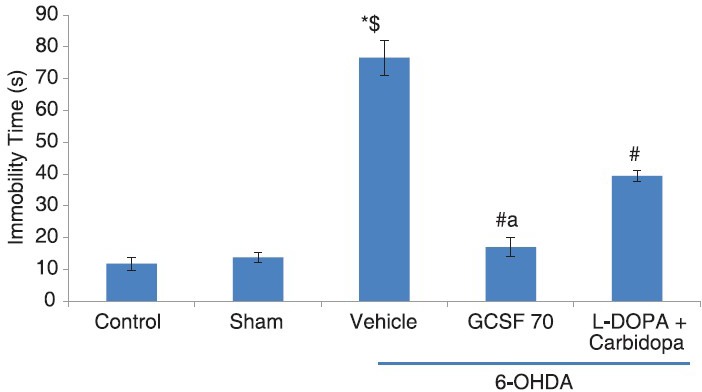
Immobility test, effect of granulocyte-colony stimulating factor on FST test in 6-hydroxydopamine (6-OHDA) induced Parkinson's disease model of rats, *P< 0.05, as compared to control; $P< 0.05, as compared to sham; #P< 0.05, as compared to 6-OHDA + vehicle group, aP< 0.05, as compared to L-DOPA/carbidopa
Neuronal Density
Nissl staining for the SNc neuron showed significantly loss in vehicle treated group as compared to control and sham operated group. However, G-CSF 70 showed significantly increased neuronal density in SNc region of rat brain when compared with the vehicle as well as L-DOPA + carbidopa group [Figure 3].
Figure 3.
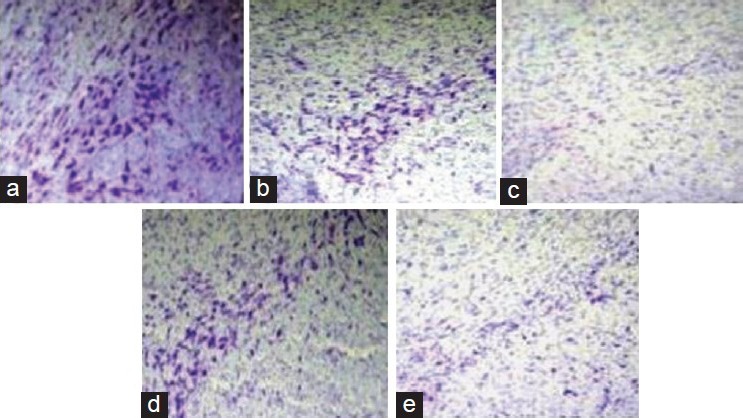
Nissl staining, effect of granulocyte-colony stimulating factor (G-CSF) on neuronal density in substantia nigra pars compacta in 6-hydroxydopamine induced Parkinson's disease model of rats, (a) control group, (b) sham operated group, (c) vehicle treated group (d) G-CSF 70 and (e) L-DOPA + carbidopa
Discussion
The present study is a preliminary one, aimed to show the neuroprotective effect of G-CSF in PD rat model. This is the first study, which implicated the effect of G-CSF on PD associated with depression. Present study showed that G-CSF significantly reduced the adhesive removal time and immobility time in FST test. Neuronal density was also found to increase highly in SNc region of the brain.
Among the model of PD, unilateral 6-OHDA model is frequently used in rats and has the advantage of presenting side-biased motor impairments and produced symptoms like PD. As the finding of the present study rats 6-OHDA significantly increased the reaction time to remove the adhesive. Bouet et al.[19] 2009 showed that adhesive removal test is of have high sensitivity, because it showed coordination behavior of paw and mouth. Hence, this test gives correct outcome of sensitivity such as time to contact paw and mouth and correct dexterity, i.e., time to remove. The impaired behavior was found to improve after the G-CSF treatment.
Cummings,[2] 1992 reported that depression occurs in approximately 40% of patients with PD and it is distinguished from other depressive disorders by greater anxiety and less self-punitive ideation. FST is a very commonly used animal model to assess the depression like behavior. FST showed significantly increased immobility time in vehicle treated rats, whereas G-CSF treatment significantly reduced the immobility time. The neuroprotective activity of G-CSF is significantly supported by the increased neuronal density in SNc. Similarly, Song et al.[20] 2011, reported that G-CSF enhances recovery of DA nigro-striatal function from MPTP toxicity in part by modulating the microglial response to injury. However, other studies reported that neuroprotective effects of G-CSF have been attributed to activation of several anti-apoptotic pathways. G-CSF prevented neuronal death triggered by methyl-phenylpyridinium in cell cultures of primary midbrain neurons and PC12 cells by increasing expression of the anti-apoptotic protein Bcl-2 while decreasing the pro-apoptotic Bax.[21,22] Activation of the PI3K/Akt pathway by G-CSF in cultured rat cortical neurons was reported to be another anti-apoptotic pathway responsible for neuroprotection.[23]
Conclusion
The present study showed that G-CSF have a potential role in the treatment of PD associated with depression. However, this is a preliminary part of study and has required more molecular assessment to conclude the finding of the present study.
Footnotes
Source of Support: Nil
Conflict of Interest: No
References
- 1.Siderowf A, Stern M. Update on Parkinson disease. Ann Intern Med. 2003;138:651–8. doi: 10.7326/0003-4819-138-8-200304150-00013. [DOI] [PubMed] [Google Scholar]
- 2.Cummings JL. Depression and Parkinson's disease: A review. Am J Psychiatry. 1992;149:443–54. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Forno LS. Neuropathology of Parkinson's disease. J Neuropathol Exp Neurol. 1996;55:259–72. doi: 10.1097/00005072-199603000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Schrag A, Jahanshahi M, Quinn N. How does Parkinson's disease affect quality of life? A comparison with quality of life in the general population. Mov Disord. 2000;15:1112–8. doi: 10.1002/1531-8257(200011)15:6<1112::aid-mds1008>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 6.Schrag A. Quality of life and depression in Parkinson's disease. J Neurol Sci. 2006;248:151–7. doi: 10.1016/j.jns.2006.05.030. [DOI] [PubMed] [Google Scholar]
- 7.Antonini A. Unraveling depression in Parkinson's disease. Eur J Neurol. 2008;15:885–6. doi: 10.1111/j.1468-1331.2008.02216.x. [DOI] [PubMed] [Google Scholar]
- 8.Prado RC, Barbosa ER. Depression in Parkinson's disease: Study of 60 cases. Arq Neuropsiquiatr. 2005;63:766–71. doi: 10.1590/s0004-282x2005000500009. [DOI] [PubMed] [Google Scholar]
- 9.Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, et al. Depression rating scales in Parkinson's disease: Critique and recommendations. Mov Disord. 2007;22:1077–92. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grigg AP, Roberts AW, Raunow H, Houghton S, Layton JE, Boyd AW, et al. Optimizing dose and scheduling of filgrastim (granulocyte colony-stimulating factor) for mobilization and collection of peripheral blood progenitor cells in normal volunteers. Blood. 1995;86:4437–45. [PubMed] [Google Scholar]
- 11.Khan M, Sekhon B, Giri S, Jatana M, Gilg AG, Ayasolla K, et al. S-Nitrosoglutathione reduces inflammation and protects brain against focal cerebral ischemia in a rat model of experimental stroke. J Cereb Blood Flow Metab. 2005;25:177–92. doi: 10.1038/sj.jcbfm.9600012. [DOI] [PubMed] [Google Scholar]
- 12.Komine-Kobayashi M, Zhang N, Liu M, Tanaka R, Hara H, Osaka A, et al. Neuroprotective effect of recombinant human granulocyte colony-stimulating factor in transient focal ischemia of mice. J Cereb Blood Flow Metab. 2006;26:402–13. doi: 10.1038/sj.jcbfm.9600195. [DOI] [PubMed] [Google Scholar]
- 13.Gibson CL, Jones NC, Prior MJ, Bath PM, Murphy SP. G-CSF suppresses edema formation and reduces interleukin-1beta expression after cerebral ischemia in mice. J Neuropathol Exp Neurol. 2005;64:763–9. doi: 10.1097/01.jnen.0000179196.10032.dd. [DOI] [PubMed] [Google Scholar]
- 14.Vernon AC, Palmer S, Datla KP, Zbarsky V, Croucher MJ, Dexter DT. Neuroprotective effects of metabotropic glutamate receptor ligands in a 6-hydroxydopamine rodent model of Parkinson's disease. Eur J Neurosci. 2005;22:1799–806. doi: 10.1111/j.1460-9568.2005.04362.x. [DOI] [PubMed] [Google Scholar]
- 15.Chan H, Paur H, Vernon AC, Zabarsky V, Datla KP, Croucher MJ, et al. Neuroprotection and Functional Recovery Associated with Decreased Microglial Activation Following Selective Activation of mGluR2/3 Receptors in a Rodent Model of Parkinson's Disease. Parkinsons Dis 2010. 2010 doi: 10.4061/2010/190450. pii: 190450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freret T, Bouet V, Leconte C, Roussel S, Chazalviel L, Divoux D, et al. Behavioral deficits after distal focal cerebral ischemia in mice: Usefulness of adhesive removal test. Behav Neurosci. 2009;123:224–30. doi: 10.1037/a0014157. [DOI] [PubMed] [Google Scholar]
- 17.Porsolt RD, Bertin A, Jalfre M. Behavioral despair in mice: A primary screening test for antidepressants. Arch Int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- 18.Burke RE, Macaya A, DeVivo D, Kenyon N, Janec EM. Neonatal hypoxic-ischemic or excitotoxic striatal injury results in a decreased adult number of substantia nigra neurons. Neuroscience. 1992;50:559–69. doi: 10.1016/0306-4522(92)90447-a. [DOI] [PubMed] [Google Scholar]
- 19.Bouet V, Boulouard M, Toutain J, Divoux D, Bernaudin M, Schumann-Bard P, et al. The adhesive removal test: A sensitive method to assess sensorimotor deficits in mice. Nat Protoc. 2009;4:1560–4. doi: 10.1038/nprot.2009.125. [DOI] [PubMed] [Google Scholar]
- 20.Song S, Sava V, Rowe A, Li K, Cao C, Mori T, et al. Granulocyte-colony stimulating factor (G-CSF) enhances recovery in mouse model of Parkinson's disease. Neurosci Lett. 2011;487:153–7. doi: 10.1016/j.neulet.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Cao XQ, Arai H, Ren YR, Oizumi H, Zhang N, Seike S, et al. Recombinant human granulocyte colony-stimulating factor protects against MPTP-induced dopaminergic cell death in mice by altering Bcl-2/Bax expression levels. J Neurochem. 2006;99:861–7. doi: 10.1111/j.1471-4159.2006.04125.x. [DOI] [PubMed] [Google Scholar]
- 22.Meuer K, Pitzer C, Teismann P, Krüger C, Göricke B, Laage R, et al. Granulocyte-colony stimulating factor is neuroprotective in a model of Parkinson's disease. J Neurochem. 2006;97:675–86. doi: 10.1111/j.1471-4159.2006.03727.x. [DOI] [PubMed] [Google Scholar]
- 23.Schneider A, Krüger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–98. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]


