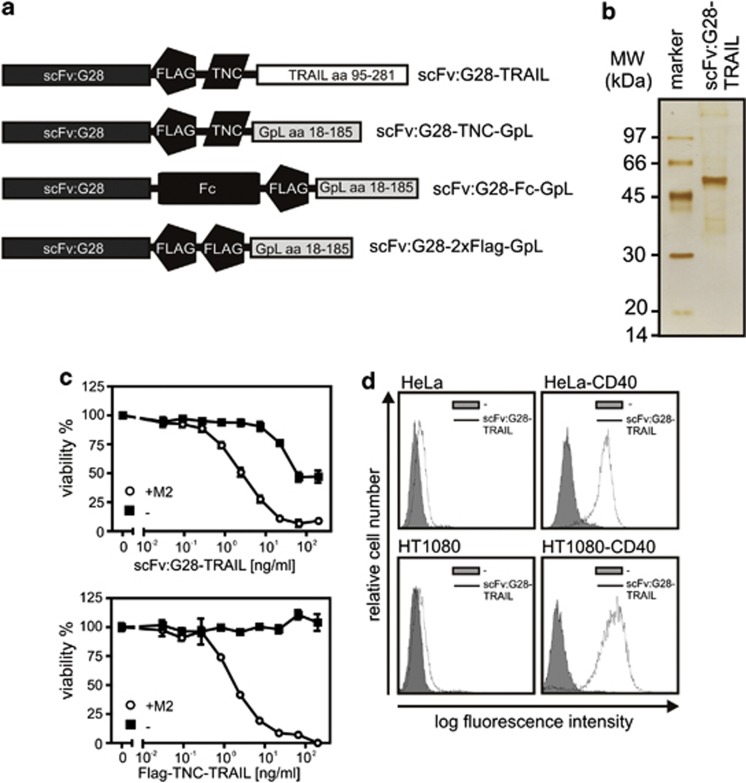
Figure 1.
Domain architecture of the scFv:G28 fusion proteins used in this study and initial analysis of scFv:G28-TRAIL. (a) Schemes of scFv:G28-TRAIL, scFv:G28-2xFlag-GpL, scFv:G28-Fc-GpL and scFv:G28-TNC-GpL. scFv:G28, scFv derived from the human CD40-specific mAb G28-5; Flag epitope; TNC, trimerization domain, aa 110-139 of chicken tenascin-C; TRAIL, aa 95-281 of human TRAIL; Fc, CH3 domain of hIgG1; GpL, luciferase of Gaussia princeps. scFv:G28-Fc-GpL and scFv:G28-2xFlag-GpL were used as competitors for scFv:G28-TRAIL. scFv:G28-TNC-GpL were used to analyze the effect of trimerization of scFv:G28 on CD40 activity. (b) Anti-Flag affinity chromatography purified scFv:G28-TRAIL (100 ng) was subjected to SDS-PAGE and visualized by silver staining. (c) Jurkat cells (60 × 103 per well; 96-well plate) were challenged in the absence and presence of the anti-Flag mAb M2 (1 μg/ml) with the indicated concentrations of scFv:G28-TRAIL or its conventional counterpart Flag-TNC-TRAIL. Next day, cellular viability was determined by using the MTT assay (d) HeLa and HT1080 cells along with the corresponding CD40 transfectants were incubated with scFv:G28-TRAIL (500 ng/ml) and after three washes, bound molecules were detected by FACS using anti-Flag mAb M2-FITC