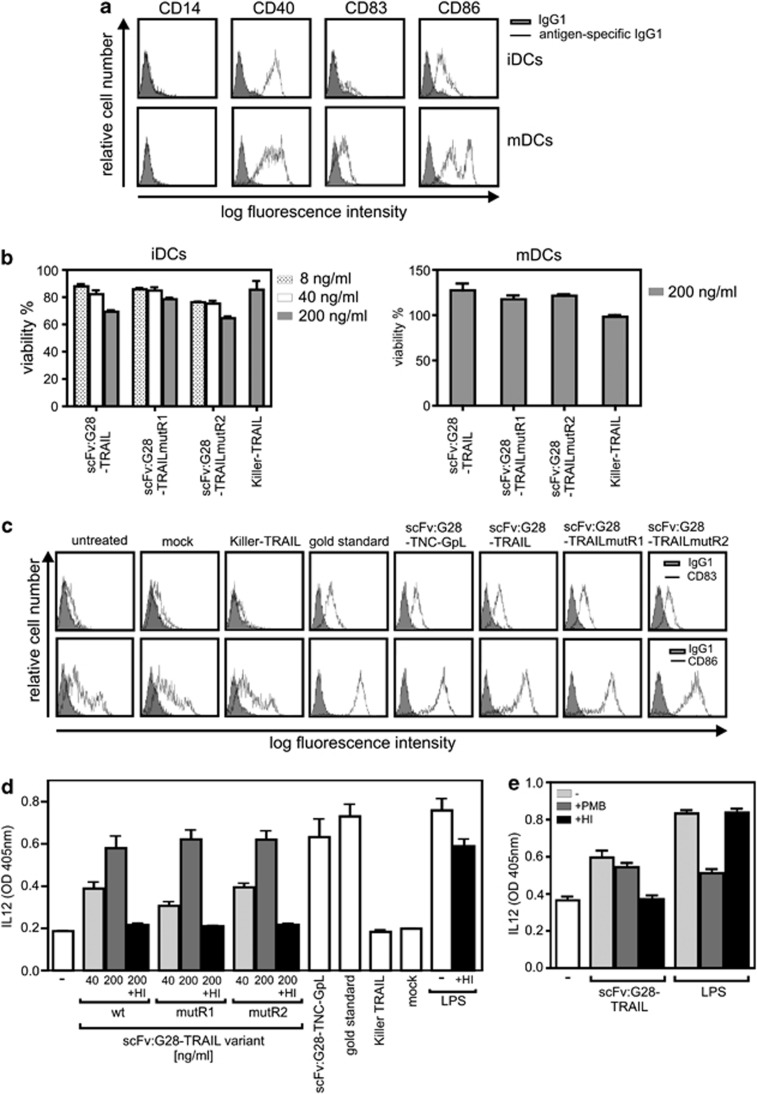
Figure 8.
scFv:G28-TRAIL fusion proteins stimulate DC maturation. (a) Immature DCs (iDCs) and DCs (mDCs) that have been matured with the gold standard (mixture of 20 ng/ml TNF, 10 ng/ml IL1β, 20 ng/ml IL-6 and 1 μg/ml PGE2) were analyzed by FACS with respect to the cell surface expression of the indicated proteins. (b) iDCs and mDCs were challenged with scFv:G28-TRAIL variants, Killer-TRAIL or a cytotoxic cocktail (100 ng/ml Fc-CD95L, 5 μg/ml CHX and 0.5% sodium azide). Next day, cellular viability was determined using the MTT assay and normalized according to cells treated with the cytotoxic cocktail. (c) iDCs were treated for 48 h with 200 ng/ml of the various scFv:G28-TRAIL variants, with 200 ng/ml scFv:G28-TNC-GpL, a tenascin-C trimerization domain-containing fusion protein of scFv:G28 and the Gaussia princeps luciferase or with Killer-TRAIL (200 ng/ml). A supernatant of mock transfected HEK293 cells were included as a negative control for scFv:G28-TNC-GpL. To evaluate DC maturation, cells were analyzed by FACS with respect to the cell surface expression of CD83 and CD86 and compared with untreated groups and DCs were incubated with the ‘gold standard' mixture. (d) iDCs were treated in triplicates with the indicated concentrations of the various scFv:G28-TRAIL variants as well as with Killer-TRAIL (200 ng/ml), the gold standard mixture, LPS (100 ng/ml), scFv:G28-TNC-GpL and a control supernatant of mock transfected HEK293 cells. Where indicated, samples were heat-inactivated (HI) at 70 °C for 30 min to check for heat-stable endotoxin contaminations. DC supernatants were assayed after 24 h for the production of IL12 by ELISA. (e) iDCs were treated in triplicates with 200 ng/ml scFv:G28-TRAIL or LPS (20 ng/ml). Where indicated, samples were HI at 70 °C for 30 min or treated with 50 μg/ml polymyxin B (PMB). After 24 h, DC supernatants were again assayed for IL12 production by ELISA