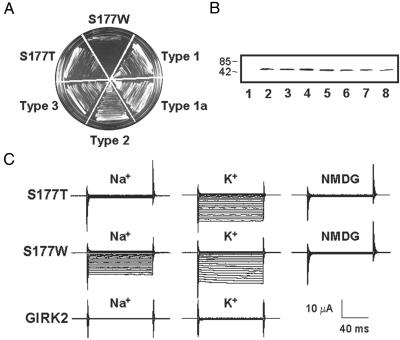
Fig. 1.
S177W mutation in M2 abolishes K+ selectivity of GIRK2. (A) Wild-type GIRK2 did not rescue yeast growth due to low probability of opening in the absence of mammalian Gβγ. Constitutively active but nonselective S177W GIRK2 failed to rescue yeast growth on a plate supplemented with a low concentration of K+ (0.5 mM KCl), whereas K+-selective S177T GIRK2 supported yeast growth, as did four mutant types each containing multiple mutations in addition to S177W. (B) Comparable channel protein-expression levels of wild type (lane 2), S177T (lane 3), S177W (lane 4), and the four mutant types (lanes 5–8) in yeast. The predicted mass of the GIRK2 monomer is 47.5 kDa. Anti-GIRK2 antibody does not recognize Kir2.1 (IRK1) channel expressed in the same yeast strain (lane 1). (C) Representative current traces obtained from Xenopus oocytes expressing wild-type GIRK2, S177T, or S177W mutant channels bathed in 90 mM K+, 90 mM Na+, or 90 mM NMDG. Currents were recorded at membrane potentials ranging from +40 to –150 mV in 10-mV increments.