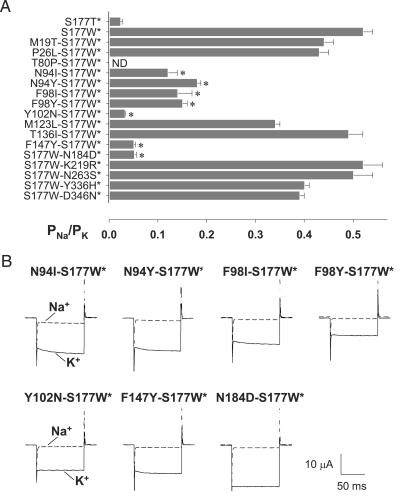
Fig. 3.
Seven mutations of five residues in the transmembrane domain partially restore K+ selectivity of the nonselective S177W channel. (A) The PNa/PK permeability ratio of S177W-containing double mutants for each of the mutations included in the four mutant types. Seven mutations, marked with asterisks (*) in the bar graph, reduced PNa/PK by >50%. ND indicates that PNa/PK could not be determined because the current was indistinguishable from leak current. (B) Suppression of the Na+ current caused by the S177W mutation by second-site suppressors. Current traces were elicited with voltage pulses from +40 to –150 mV (in 10-mV increments) from a holding potential of 0 mV in 90 mM K (solid line) and 90 mM Na (dashed line). Only traces at –150 mV are shown. Whereas currents recorded in 90 mM K solution from “double mutants” (in the background of the D228N mutation) were comparable in amplitude, some exhibited a slower time course of current activation.