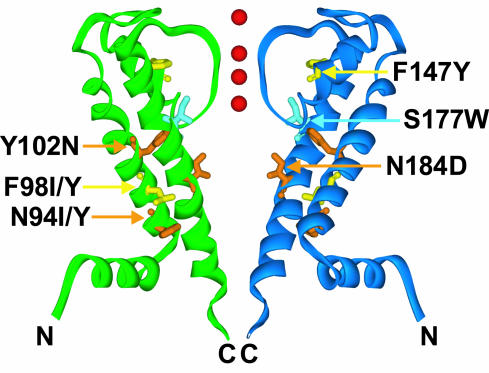
Fig. 4.
Suppressors are located on helices in the transmembrane domain of GIRK2. Suppressors are mapped onto the KirBac1.1 structure in two opposing KirBac monomers. S177W (pale blue), affecting a pore-lining residue of the inner helix M2, abolishes K+ selectivity. Suppressors N94Y/I, F98Y/I, and Y102N affect M1 residues, F147Y affects a pore–helix residue, and N184D affects a pore-lining residue in M2. F98Y/I and F147Y mutations (yellow) restored K+ selectivity to S177W-containing channels, as did N94Y/I, Y102N, and N184D mutations (orange), which also impacted channel gating. Red spheres represent K+ ions in the pore.